Cytochalasin D
Details
Specifications
Chemical identification
Synonyms: Zygosporin A
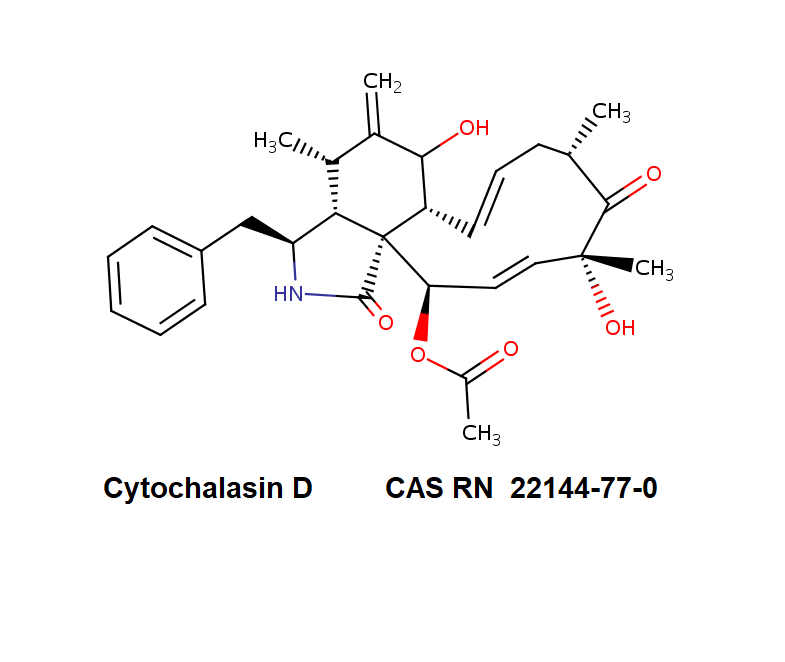
Chemical Name: (7S,13E,16S,18R,19E,21R)-21-(Acetyloxy)- 7,18-dihydroxy-16,18-dimethyl-10-phenyl[11]cytochalasa-6(12),13,19-triene-1,17-dione
Cytochalasin D is a cell permeable mycotoxin, which causes both the association and dissociation of actin subunits. Cytochalasin D disrupts actin filaments and inhibits actin polymerization.
Further Information
DMSO, Ethanol, Methanol
- Macrolide indol mycotoxin
- Cytochalasin
- Actin inhibitor
Cytochalasins are used as tools in cytological research, and in the field of actin polymerization. Cytochalasin D is 10 times more effective than cytochalasin B and does not inhibit glucose transport across cell membranes.
Composition
Special Info
Other Fields