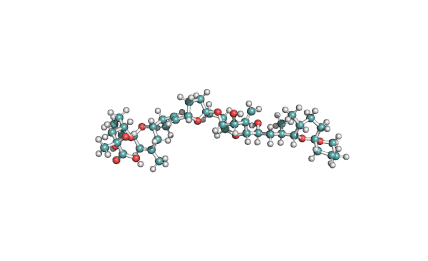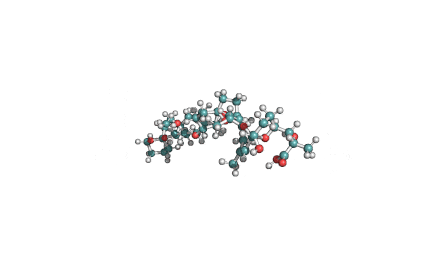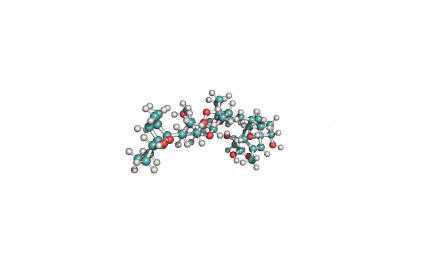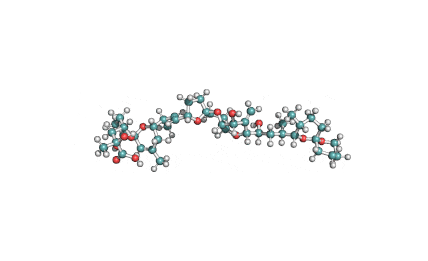
Oligomycin (complex)
Details
Specifications
Chemical identification
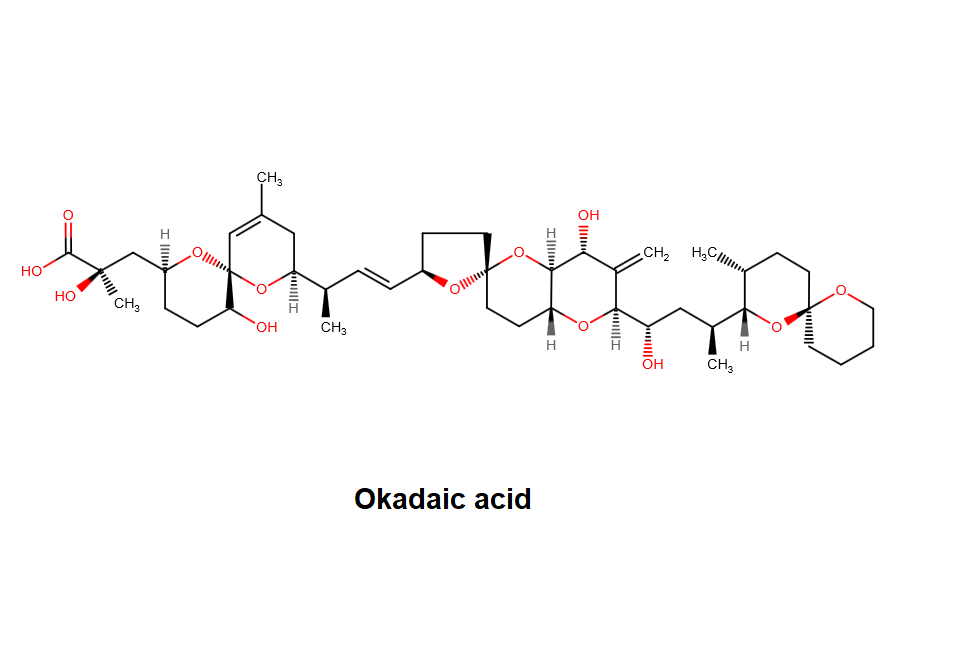
- Oligomycin (complex): a macrolide antibiotic that inhibits membrane bound mitochondrial ATPase.
- Oligomycin (complex) is a mixture of A, B, C isomers and other analogs.
- The molecular weight and molecular structure shown on this page, are of Oligomycin A, the major component.
- HPLC shows separately the concentrations of all components. Their ratio is not consistent and may vary from lot to lot. The analysis of the components is brought in CoA of particular lots.
- The three components are also offered as separate products.
Further Information
Dichloromethane, DMSO, Ethanol, Acetone
Chemical class: Macrolide
Bioactivity class: Mitochondrial ATP Synthase Inhibitor
Used as a tool in cytochemistry.
Composition
Special Info
Other Fields