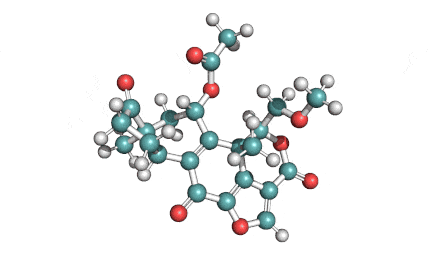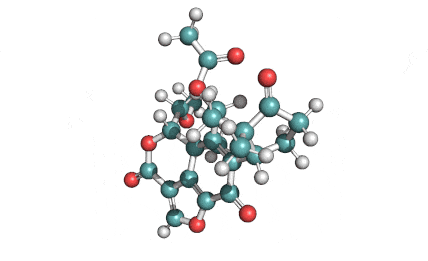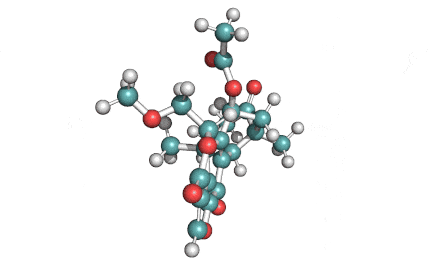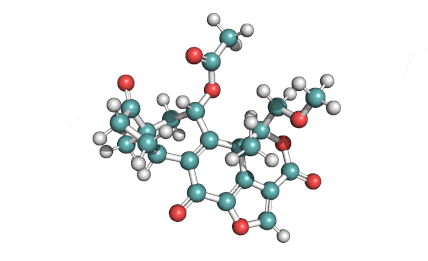
Withaferin A
Details
Specifications
Clear colorless solution at 10 mg/ml Methanol
Chemical identification
Synonyms
- Withaferin E
- 5,6-epoxy-4,22,27-trihydroxy-1-oxoergosta-2,24-dienoic acid delta-lactone
RTECS: KE7288500
Withaferin A is a cell-permeable steroidal lactone from a medicinal plant Withania somnifera, a plant known in traditional Indian medicine.
Further Information
Methanol, Ethanol, DMSO
- Ergosterol analog
- inhibitor of angiogenesis
Withaferin A has shown significant anticancer activity in animal studies. Withaferin A is an angiogenesis inhibitor . Withaferin A has immunomodulating properties. Withaferin A has radiosensitizing effect on cancer cells. Withaferin A has been reported to induce neuronal regeneration.
Composition
Special Info
Other Fields