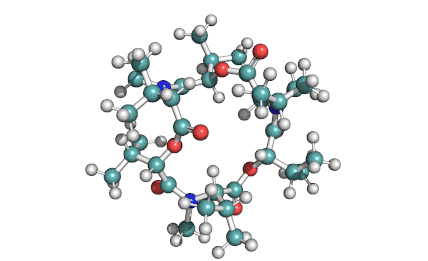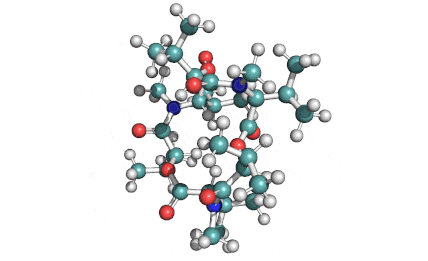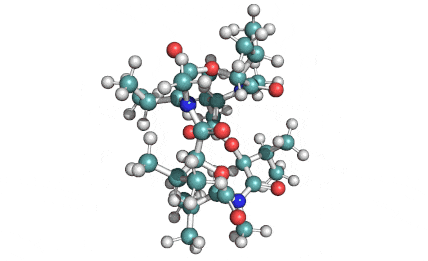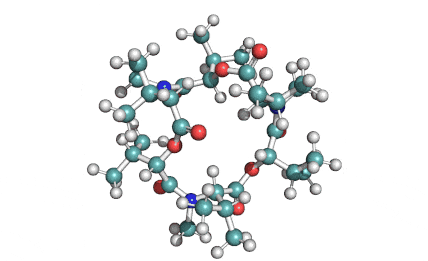
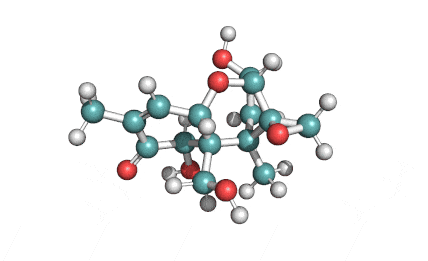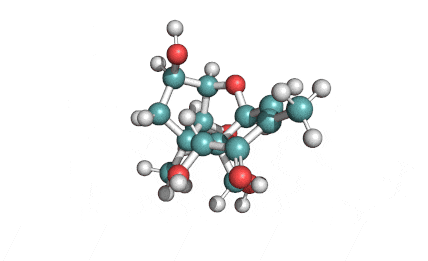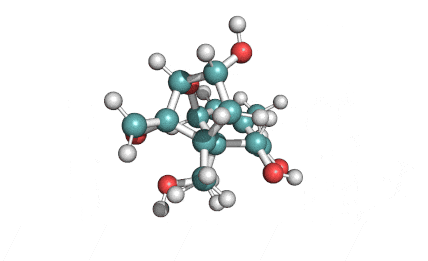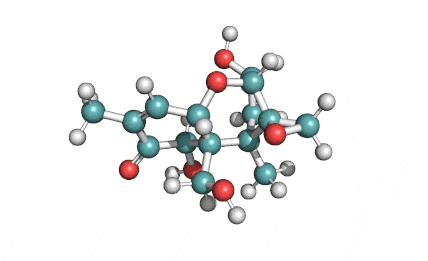
Cylindrospermopsin
Details
Specifications
Chemical identification
Synonyms:
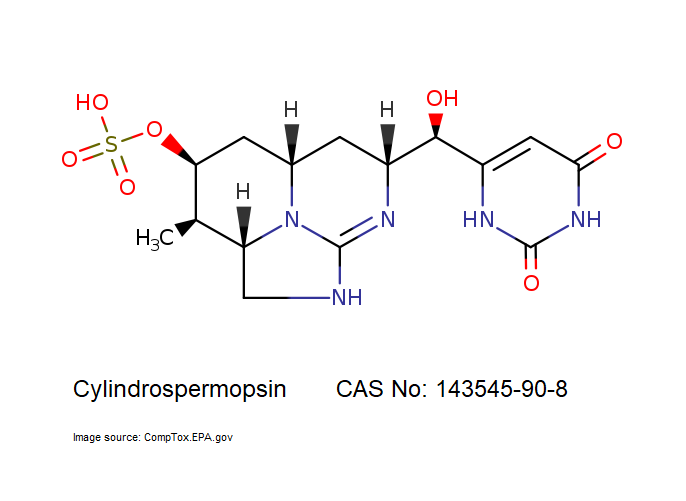
- Cylindrospermopsin
- 6-[(R)-Hydroxy[(2aS,3R,4S,5aS,7R)-2,2a,3,4,5,5a,6,7-octahydro-3-methyl-4-(sulfooxy)-1H-1,8,8b-triazaacenaphthylen-7-yl]methyl]-2,4(1H,3H)-pyrimidinedione
Chemical names:
IUPAC: (2aS,3R,4S,5aS,7R)-7-[(R)-(2,6-Dioxo-1,2,3,6-tetrahydro-4-pyrimidinyl)(hydroxy)methyl]-3-methyl-2,2a,3,4,5,5a,6,7-octahydro-1H-1,8,8b-triazaacenaphthylen-4-yl hydrogen sulfate
RTECS: UV9104310
Tricyclic alkaloid hepatotoxin from a cyanobacterium.
Cylindrospermopsin (abbreviated to CYN, or CYL) is a cyanotoxin produced by a variety of freshwater cyanobacteria. CYL is a polycyclic uracil derivative containing guanidino and sulfate groups. It is also zwitterionic, making it highly water soluble. CYL is toxic to liver and kidney tissue and is thought to inhibit protein synthesis and to covalently modify DNA and/or RNA.
It is not known whether cylindrospermopsin is a carcinogen, but it appears to have no tumour initiating activity in mice. (source: T3DB )
Further Information
Composition
Supply related information
Special Info
Other Fields