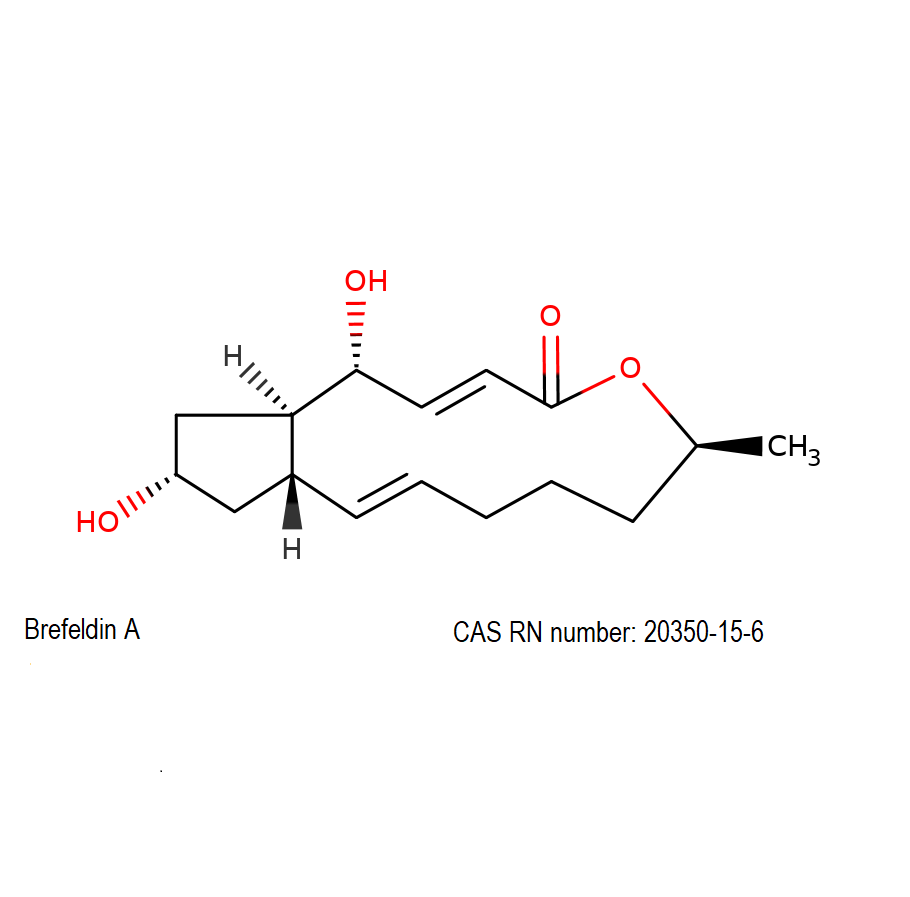
Brefeldin A
Details
Specifications
Chemical identification
Synonyms:
- Nectrolide
- Synergisidin
- Cyanaein
- Ascotoxin
- Cyanein
- Decumbin
- Brefeldin A
EC# : 606-528-3
Systematic name:
(1R,2E,6S,10E,11aS,13S,14aR)-1,13-Dihydroxy-6-methyl-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f]oxacyclotridecin-4-one
RTECS: GY8410000
Brefeldin A is a macrocyclic lactone from fungal source, exhibiting a wide range of antibiotic activity.
Further Information
Methanol, Ethanol, Dichloromethane, DMSO, acetone or ethyl acetate
- Macrocyclic lactone antibiotic.
- Antifungal
- Antiviral
- Protein Synthesis Inhibitor
Inhibitor of protein translocation from endoplasmic reticulum (ER) to the Golgi apparatus.
Inhibitor of intracellular protein transport and protein secretionA macrolide isolated from Penicillium brefeldianum. It affects the vesticular transport of the Golgi apparatus and induces DNA fragmentation which leads to apoptosis.
Composition
Special Info
Other Fields