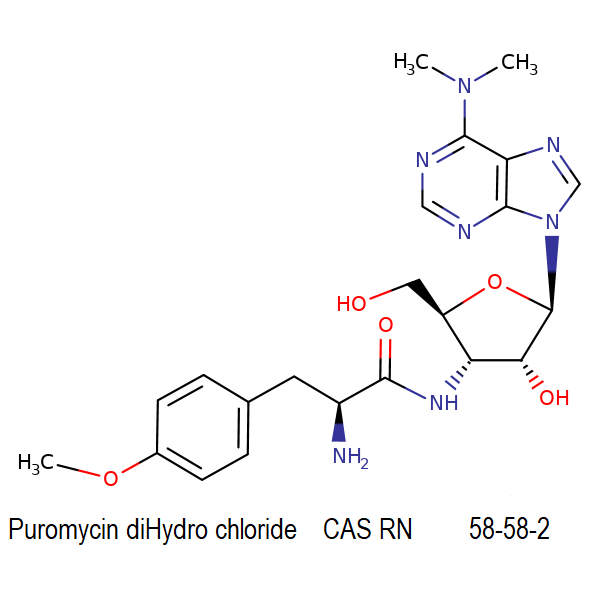
Puromycin dihydrochloride animal free
Details
Specifications
Chemical identification
Synonyms:
- Stylomycin
- Achromycin
Chemical names:
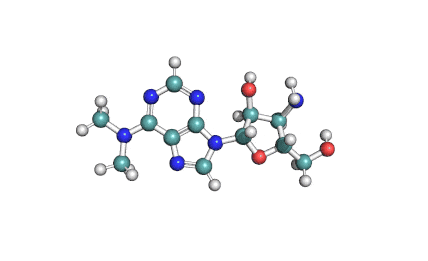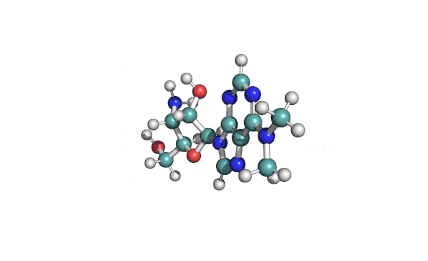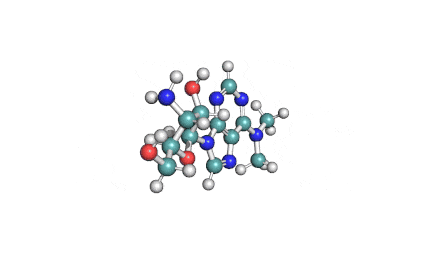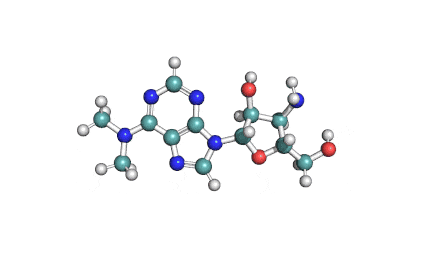
IUAPC name: 3'-deoxy-N,N-dimethyl-3'-[(O-methyl-L-tyrosyl)amino]adenosine dihydrochloride
RTECS# AU7355000
- This version of Puromycin has been prepared without using animal raw materials.
- Puromycin is a protein synthesis inhibitor. It causes premature chain termination.
Further Information
- Nucleoside antibiotic.
- Protein synthesis inhibitor
Puromycin is an antibiotic used for selecting mammalian cell lines, which have been transformed by vectors that express puromycin-N-acetyl-transferase. Puromycin is also a Antineoplastic agent. Puromycin possesses antoprotozoal activities (against Trypanozoma)
Composition
Supply related information
Special Info
Other Fields