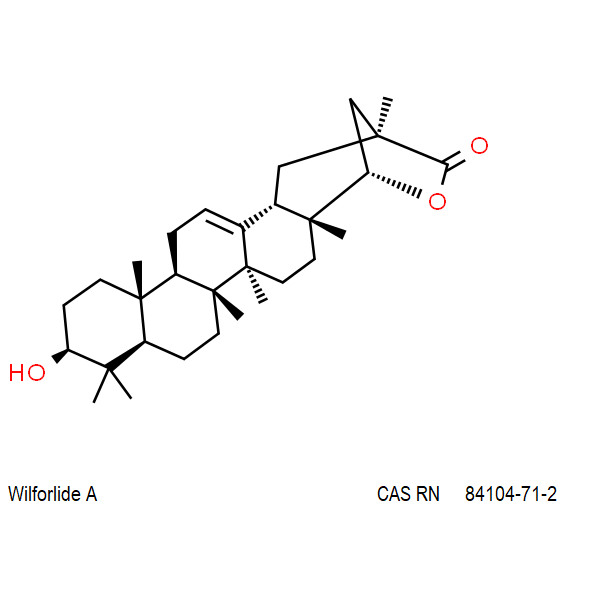
Wilforlide A
Details
Specifications
Chemical identification
Synonyms:
- Wilforlide A
- Regelide
- Abruslactone A
Chemical names:
(2S,5S,5aR,7aS,7bR,9aR,11S,13aR,13bR,15bS)-11-hydroxy-2,5a,7a,7b,10,10,13a-heptamethyl-1,5,5a,6,7,7a,7b,8,9,9a,10,11,12,13,13a,13b,14,15b-octadecahydro-2,5-methanochryseno[2,1-c]oxepin-3(2H)-one
Synonym: Wilforlide A; Regelide; Abruslactone A
IUPAC: (3β,22α)-3-Hydroxy-22,29-epoxyolean-12-en-29-one
RTECS#
Further Information
NotAnimal
No animal ingredients
NotGMO
Product natural
Health-Hazard
- Respiratory sensitization, category 1
- Germ cell mutagenicity, categories 1A, 1B, 2
- Carcinogenicity, categories 1A, 1B, 2
- Reproductive toxicity, categories 1A, 1B, 2
- Specific target organ toxicity following single exposure, categories 1, 2
- Specific target organ toxicity following repeated exposure, categories 1, 2
- Aspiration hazard, categories 1, 2
Warning or harmful
- Acute toxicity (oral, dermal, inhalation), category 4
- Skin irritation, categories 2, 3
- Eye irritation, category 2A
- Skin sensitization, category 1
- Specific target organ toxicity following single exposure, category 3
- Respiratory tract irritation
- Narcotic effects
Corrosive
- Skin corrosion, categories 1A, 1B, 1C
- Serious eye damage, category 1
Composition
Supply related information
Special Info
Other Fields