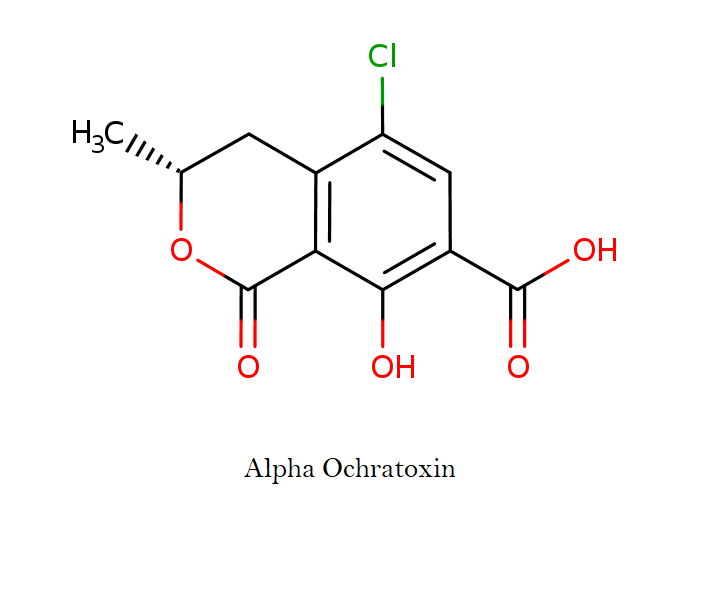
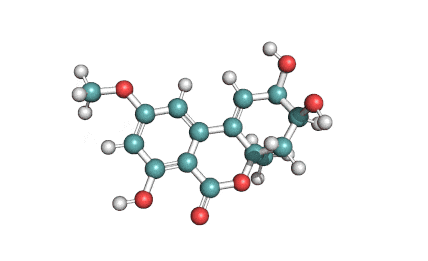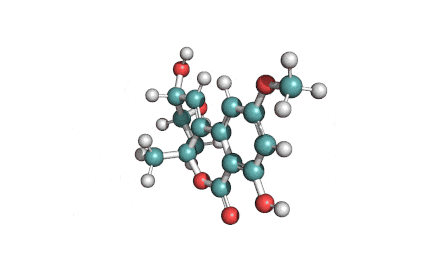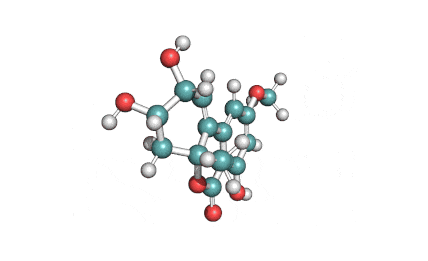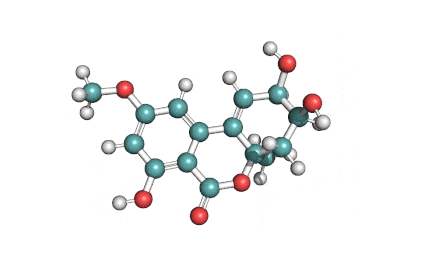
Ochratoxin alpha
Details
Specifications
Chemical identification
Synonyms:
- alpha-Ochratoxin
- Ochratoxin alpha
- 5-chloro-8-hydroxy-3,4-dihydro-3-methylisocoumarin-7-carboxylic acid
Chemical names:
IUPAC:
(3R)-5-Chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromene-7-carboxylic acid
RTECS#
Nontoxic metabolite of Ochratoxin A
Further Information
OTalpha (OTα) formed by the cleavage of the peptidic bond in OTA is a major metabolite not only in animals and humans, but also in microorganisms and enzyme systems. It is considered as a nontoxic product.
Composition
Supply related information
Other Fields