Standard Solution Fumonisin B1,B2 mixture
Details
Chemical identification
Fumonisin B1 and Fumonisin B2 50ppm each, in Acetonitrile-water 1:1, both HPLC grade, Ready for injection. Available in 1 or 5 cc vials.
Ochratoxin
Ochratoxin A (OTA or OA) is produced by Aspergilus and Penicillium fungi. It affects the kidneys and causes both acute and chronic lesions. It is also genotoxic and affects the immune system.
Ochratoxin A is a known contaminant of cereals. Recently Ochratoxin A was found to contaminate a wide range of other stored products and processed foods including coffee, beer, dried fruit, wine, cocoa and nuts.
The EU has recently proposed statutory limits for Ochratoxin A of 5 µg/kg in raw cereal grains including rice and buckwheat, 3 µg/kg for derived cereal products or for cereal grains for direct human consumption, and 10 µg/kg in dried vine fruits.
Analytical methods used are based on TLC, HPLC or ELISA.
Fermentek offers Ochratoxin A and Ochratoxin B.
Zearalenone
Zearalenone is a mycotoxin produced by a number of Fusarium and Gibberella species that infect wheat, barley, rice, maize, sorghum, and contaminate many other agricultural products. It can survive processing in consumer products including corn and corn products, breakfast cereals, corn beer, wheat flour, bread and walnuts, as well as animal feed products.
Zearalenone causes hyperestrogenism, abortion, stillbirth's oestrus, infertility and sterility in domestic animals. Zearalenone is genotoxic and causes severe hormonal effects. It is considered a human mutagen or carcinogen and may cause human cervical cancer.
Zearalenone was evaluated by JECFA (Joint FAO/WHO Expert Committee on Food Additives) which established a temporary TDI (Tolerable Daily Intake) of 0.2 µg/kg of body weight per day. Estimates of average dietary intakes for some ‘European’ consumers were of the order of 0.02 µg/kg of body weight per day suggesting a significant margin of safety based on current knowledge.
TLC methods have been used for Zearalenone analysis but are now superseded by HPLC with UV or fluorescence detection, GC/ECD, GC/MS and HPLC/MS.
Patulin
Patulin is produced by Penicillium, Aspergillus and Byssochlamys species growing on apples, pears, grapes and other fruit. The principal risk arises when contaminated fruit is used for the production of juices and other processed products. The most important threat of Patulin for humans is in apples and in apple juice, particularly that produced by direct pressing of apples.
Patulin causes nausea, vomiting, gastrointestinal hyperaemia, distension, haemorrhage, and ulceration. It has also been shown to be immunotoxic and neurotoxic.
Codex Committees focusing on Food Additives and Contaminants, have established limits for Patulin in apple juice and in apple juice ingredients.
The quality of fruit juice is controlled in some countries by setting a 'guideline' or 'recommended' maximum concentration agreed upon with the apple processing industry. This is commonly set at 50 µg/litre.
Patulin can be detected using TLC, but the usual method of analysis is by HPLC with UV detection.
Citrinin
Citrinin, produced by Penicillium fungi is associated with yellow rice fever in the Far East. Citrinin is found in rice, wheat, flour, barley, maize, rye, oats, peanuts and fruit, and can cause kidney and liver damage to humans as well as to animals. Other effects include vasodilation, constriction of the bronchi and increased muscular tone. Cereal samples can be screened by TLC or ELISA while quantitative results can be provided by HPLC.
Cyclopiazonic acid
Cyclopiazonic acid is produced by Penicillium and Aspergillus species. It has been detected at levels up to 10 mg/kg or higher in maize, millet, peanuts, pulses, cheese, ham, sausage, frankfurters, mixed feeds, hay, tomato, milk and other foods and feeds.
Cyclopiazonic acid appears to be toxic when present in high concentrations. It has been found to be a neurotoxin and to cause decreased weight gain, diarrhea, dehydration, depression, hyperaesthesia, hypokinesis, and convulsion in animals. Human ‘kodua poisoning’ in India from ingestion of contaminated millet seeds has been linked to cyclopiazonic acid.
A GC/MS method has been used for cyclopiazonic acid detection, while recently a HPLC/Mass Spectrometric method has been reported for the determination of cyclopiazonic acid in fungal cultures.
Additional mycotoxins
In addition to the Aflatoxins, Fumonisins, Ergots, Trichocothecenes and others mentioned above, there are additional mycotoxins that pose food safety threats. These include mycotoxins produced by Alternaria, Beauvericin, Citreoviridin, Moniliformin, Paxilline, Penicillic acid and Penitrem A.
All of these mycotoxins are produced and marketed by Fermentek.
Fumonisin B1 and Fumonisin B2 50ppm each, in Acetonitrile-water 1:1, both HPLC grade, Ready for injection. Available in 1 or 5 cc vials.
Vials containing 1 cc or 5 cc of solution of Fumonisin B3 50 ppm in Acetonitrile-water 1:1, HPLC grade, Ready for HPLC injection.
Fumonisin B2 50 ppm in Acetonitrile-water 1:1, both HPLC grade, Ready for injection. Available in 1 or 5 cc vials.
Fumonisin B1 50 ppm in Acetonitrile-water 1:1, both HPLC grade, Ready for injection. Available in 1 or 5 cc vials.
Synonyms
RTECS: FM3032000
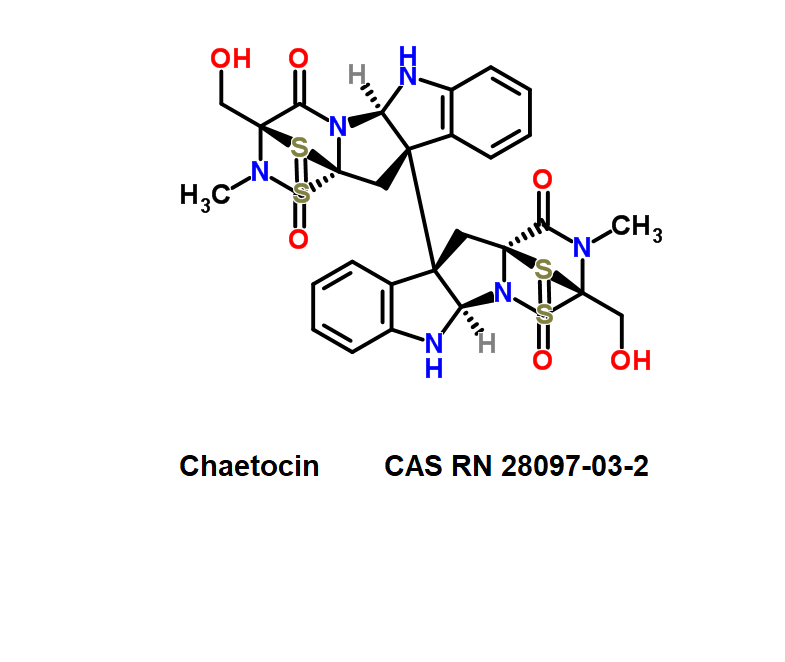
A fungal metabolite which inhibits G9a histone methyltransferase.


Synonyms
RTECS: UQ4730500
HSDB 7246
Roquefortine C is a mycotoxin produced by various fungi, particularly species from the Penicillium genus. It was first isolated from a strain of Penicillium roqueforti, a species commercially used to make Roquefort, Danish Blue, Stilton and Gorgonzola cheeses.
Soluble in 100% ethanol, methanol (10 mg/ml), DMSO (10 mg/ml), DMF, and water (poor).



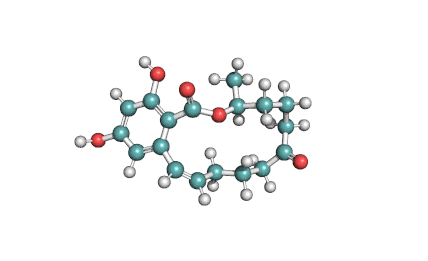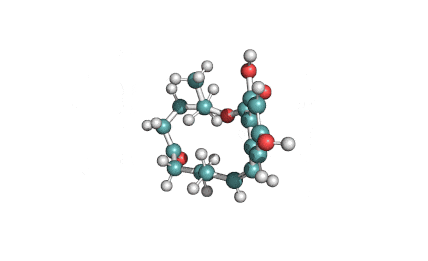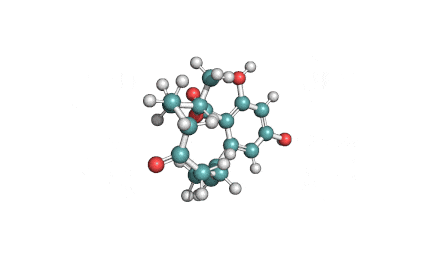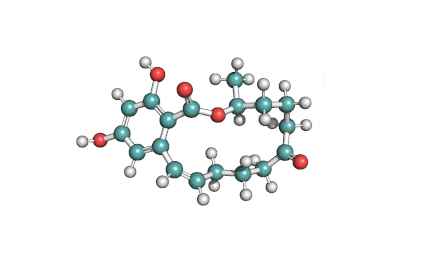
IUPAC : (3S,11E)-14,16-dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione
RTECS: DM2550000
Zearalenone is a natural estrogen, a mycotoxin from resorcylic acid lactone group.
Zearalenone is a non-steroidal estrogenic mycotoxin produced by several Fusarium spp. It has been implicated in numerous mycotoxicoses in farm animals, especially in pigs. Zearalenone is heat-stable and is found worldwide in a number of cereal crops, such as maize, barley, oats, wheat, rice, and sorghum (Kuiper-Goodman et al., 1987; Tanaka et al., 1988a) and also in bread (Aziz et al., 1997). Zearalenone was shown to be produced on corn by Fusarium isolates from Australia, Europe, and North America (Vesonder et al., 1991) and in New Zealand (diMenna et al., 1997), the Philippines, Thailand, and Indonesia (Yamashita et al., 1995). The occurrence of zearalenone in food and feed was also demonstrated in South America (Dalcero et al., 1997; Molto et al., 1997), Africa (Doko et al., 1996), China and the former USSR (Ueno et al., 1986). Fusarium isolates from bananas can also produce zearalenone
Zearalenone is slightly soluble in hexane and progressively more in benzene, acetonitrile, dichloromethane, methanol, acetone. In water: 20 mg/L .
The resorcylic acid lactones have estrogenic activity. Zearalenone and its derivates were used as veterinary anabolic or estrogen substitutes.



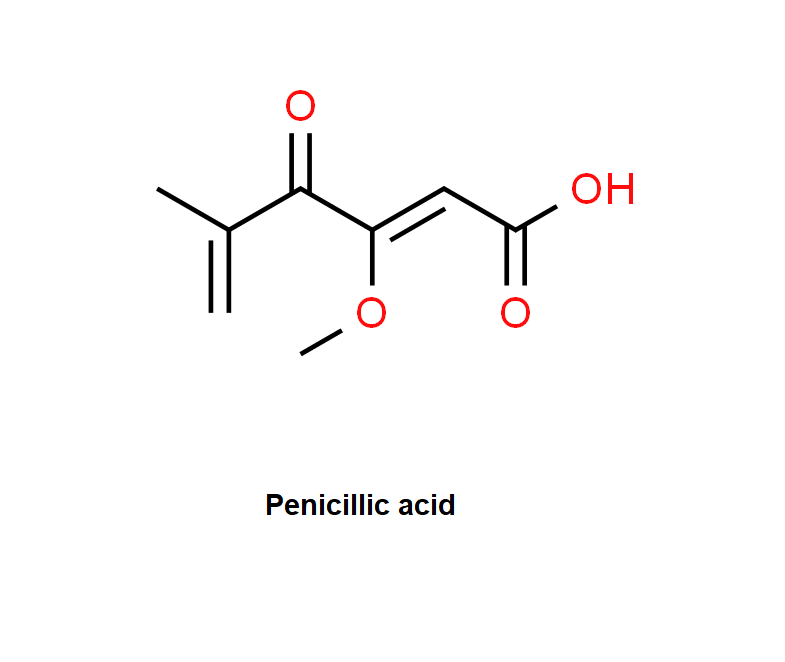
Penicillic acid is a polyketide mycotoxin which induces single and double strand DNA breaks. It inhibits GDP-mannose dehydrogenase. It inhibits also muscle aldose dehydrogenase, lactate dhydrogenase and alcohol dehydrogenase.
Water, Ethanol, Dichloromethane.
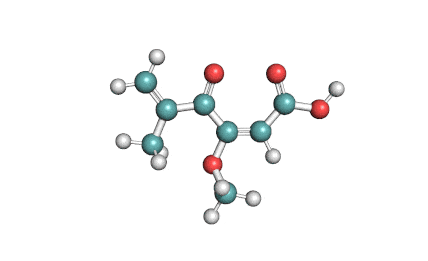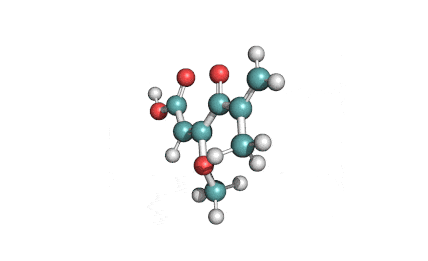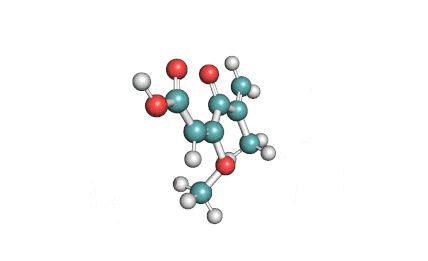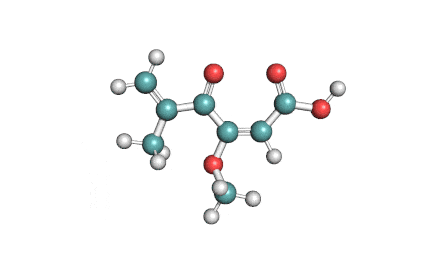


Synonyms
HSDB 3438
CHEBI 141524
RTECS SQ7331100
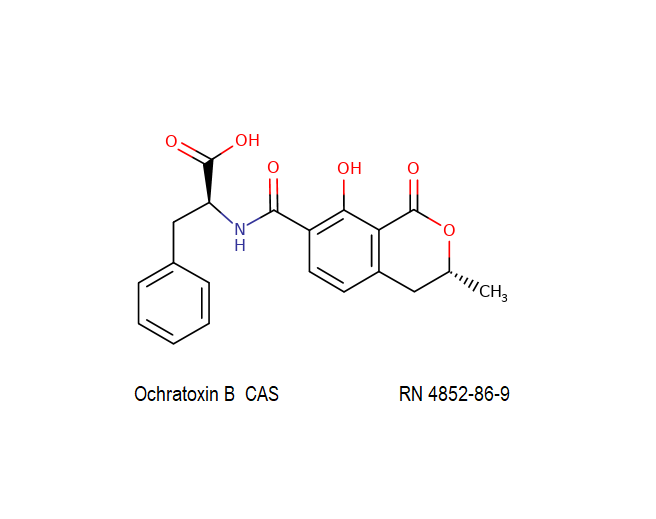
Ochratoxin B: mycotoxin; dechlorinated derivate of Ochratoxin A.
DMSO, Methanol, Ethanol, Dichloromethane.Not soluble in water
Ochratoxin B: mycotoxin. oumarin mycotoxin.
Calcium channel blocker.
Ochratoxin B is the non-chlorinated analogue of the much more extensively studied Ochratoxin A. It is co-produced by the same species of Aspergillus and Penicillium that are associated with food spoilage. Ochratoxin B has received little focused investigation. Its mode of action and its potential hazards might have been inferred from Ochratoxin-A.


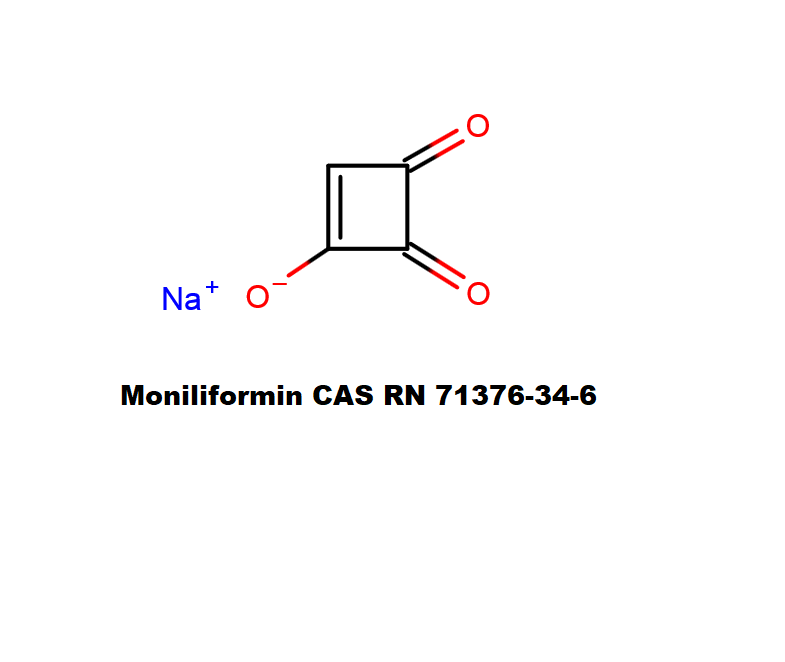
Mycotoxin formed in many cereals by a number of Fusarium species that include F.moniliforme, F. avenaceum. F. subglutinans, F. proliferatum . Moniliformin is supplied as sodium salt of 1-hydroxycyclobut-1-ene-3,4-dione.
Moniliformin is soluble in water and polar solvents, such as methanol.
cyclobutane mycotoxin