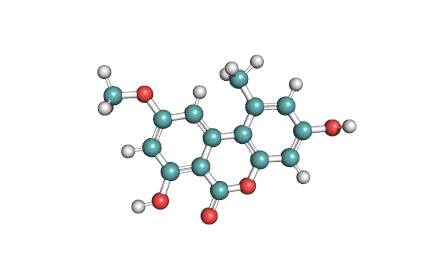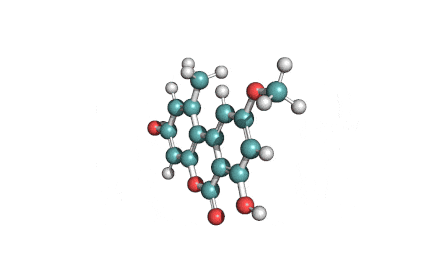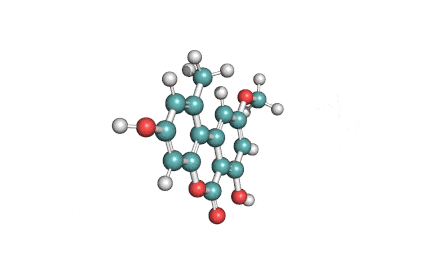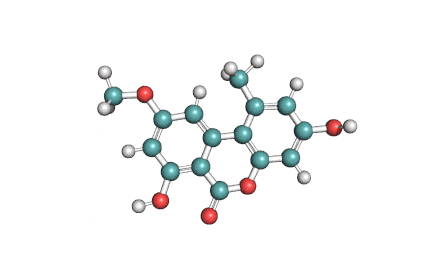
Alternariol monomethyl ether
Details
Specifications
Clear colorless solution at 1mg/ml Methanol (slight heating).
Chemical identification
Alternate names
- AME
- Djalonensone
- Alternariol monomethyl ether
- Alternariol methyl ether
- Alternariol-9-methyl ether
RTECS : HP8755000
Alternariol monomethyl ether (AME) is a natural mycotoxin originated in Alternaria alternata fungi . It inhibits Cholinesterase, and posesses antifungal and phytotoxic properties.
Further Information
Soluble in DMSO, pure ethanol, methanol
Pyrrolidinone mycotoxin
Composition
Supply related information
Special Info
Other Fields