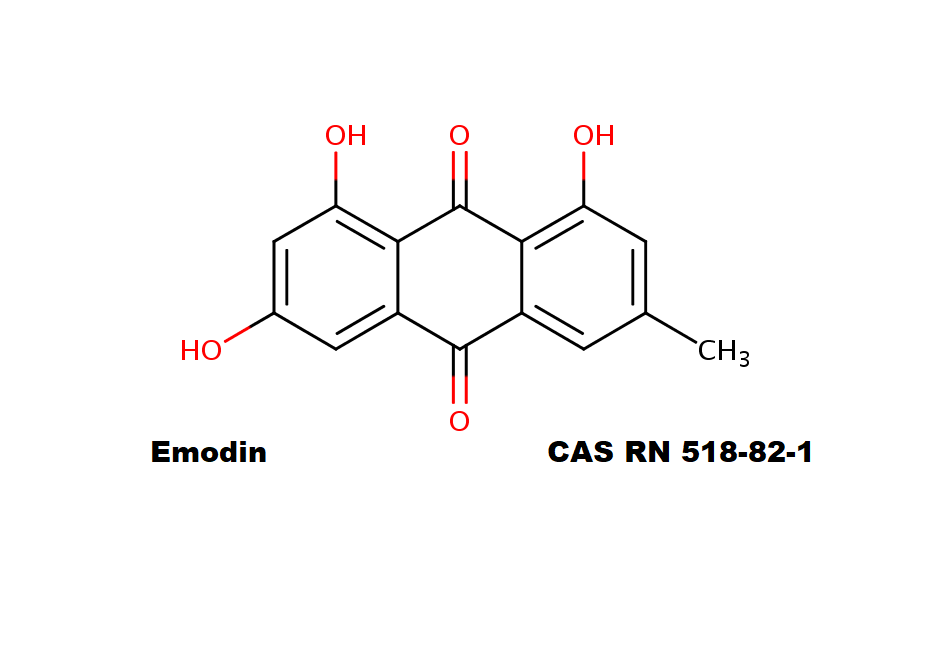
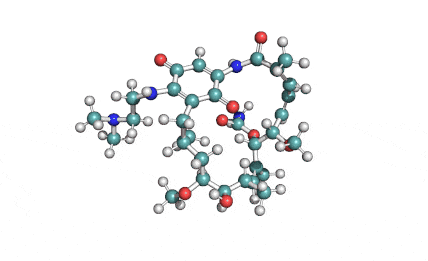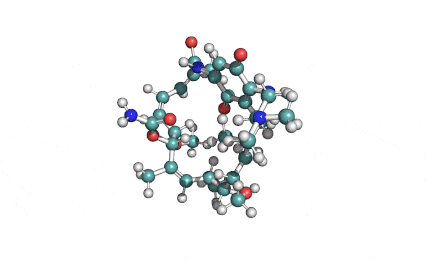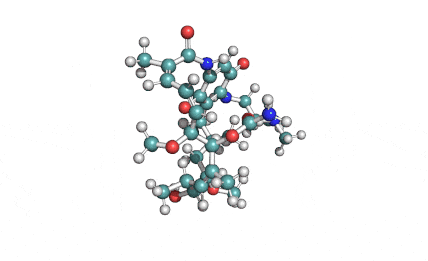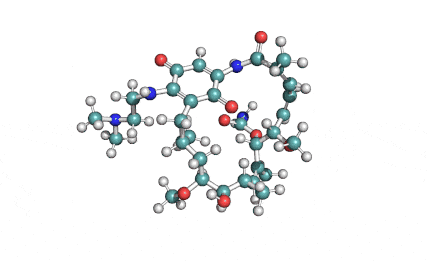
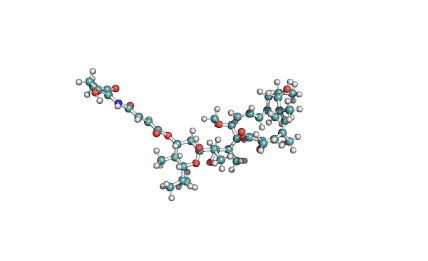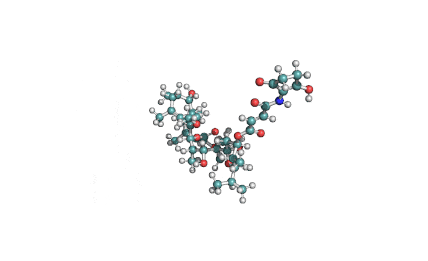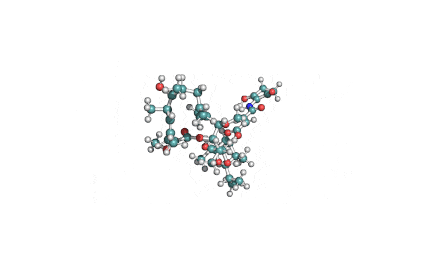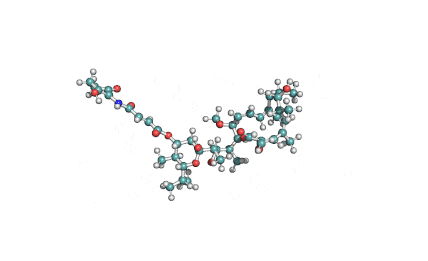
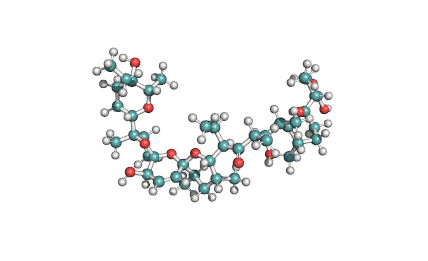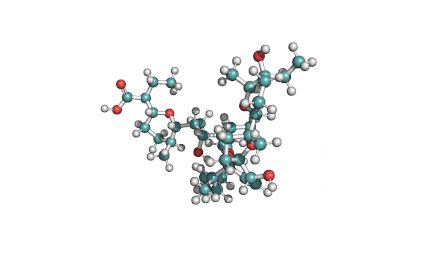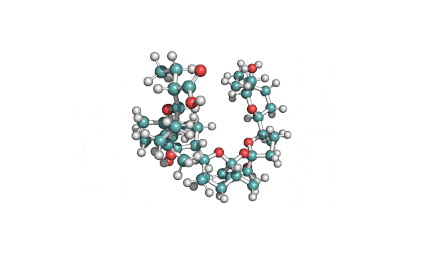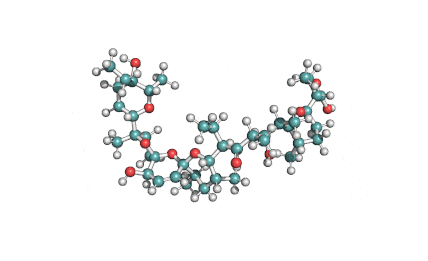
Surfactin
Details
Specifications
Chemical identification
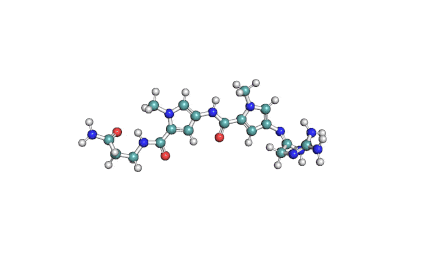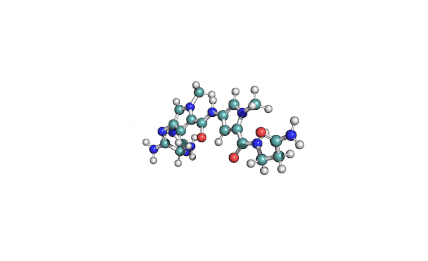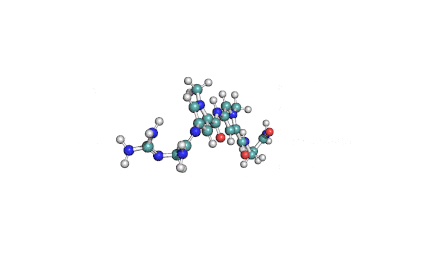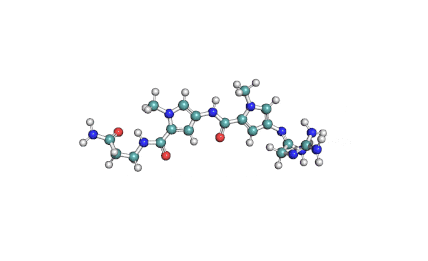
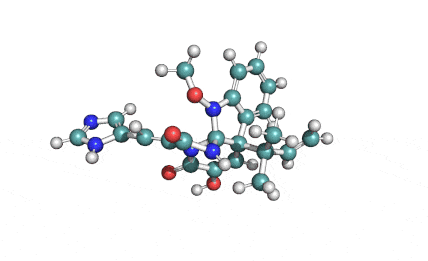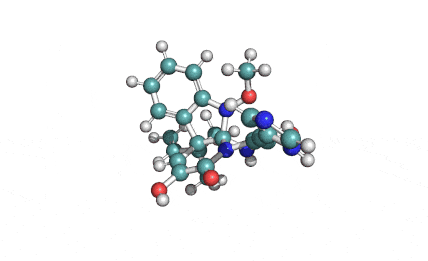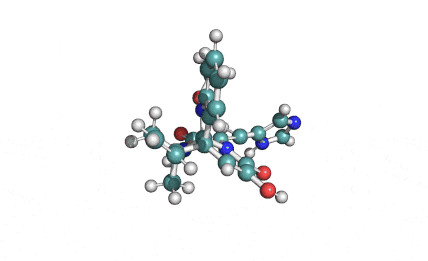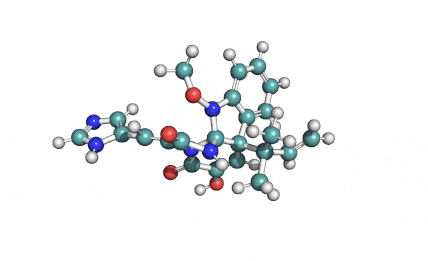
Synonyms: Cyclo(L-α-aspartyl-D-leucyl-L-leucyl-3-hydroxy-13-methyltetradecanoyl-L-α-glutamyl-L-leucyl-D-leucyl-L-valyl)
Chemical names:
IUPAC: 3-[(3S,6R,9S,12S,15R,18S,21S,25R)-9-(Carboxymethyl)-3,6,15,18-tetraisobutyl-12-isopropyl-25-(10-methylundecyl)-2,5,8,11,14,17,20,23-octaoxo-1-oxa-4,7,10,13,16,19,22-heptaazacyclopentacosan-21-yl]propanoic acid
RTECS# Not available
Disambiguation : The CAS number 24730-31-2 belongs to the isomeriso-C15-Surfactin. The product currently marketed is a mixture of several isomers. The CAS number assigned to the mixture is 252023-70-4
Surfactin is a cyclic lipopeptide from Bacillus subtilis. Surfactin posesses antibacterial, antiviral, antifungal, anti-mycoplasma and hemolytic activities. Surfactin affects the surface tension of liquids in which it is dissolved.
Further Information
Composition
Supply related information
Special Info
Other Fields