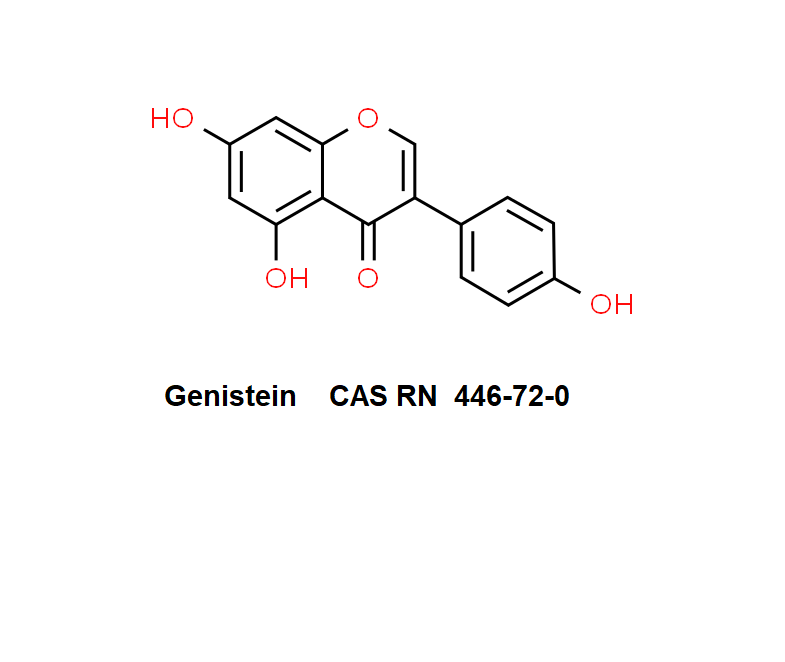
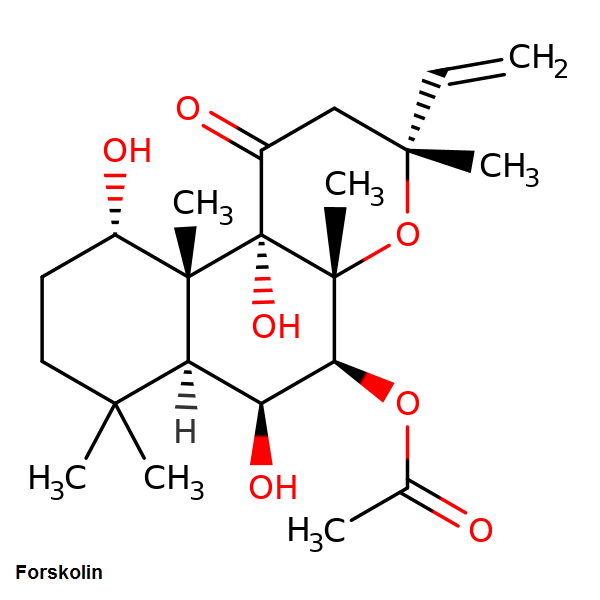
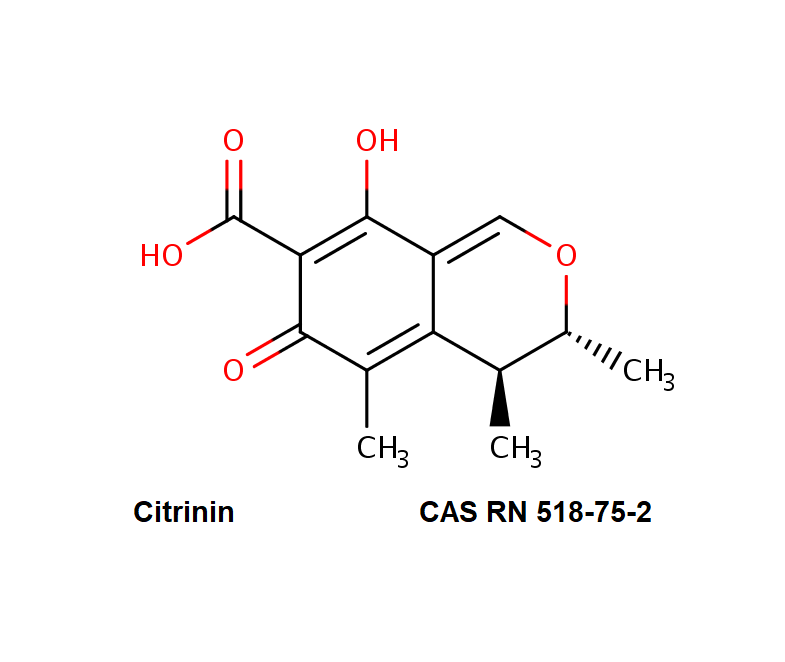
Genistein
Details
Specifications
Chemical identification
Genistein: an isoflavone with anticancer, antiproliferation, and chemopreventive effects. Genistein induces cell differentiation. Genistein inhibits protein histidine kinase.
Further Information
Genistein is soluble in DMSO, Ethanol.
isoflavonoid
histidine kinase inhibitor
Composition
Other Fields