KT5720
Details
Specifications
Chemical identification
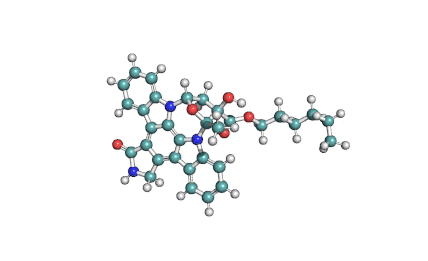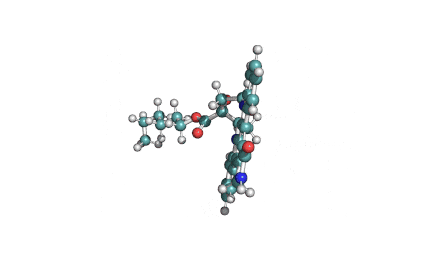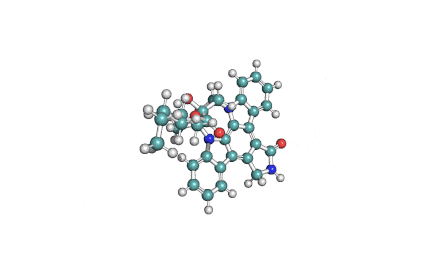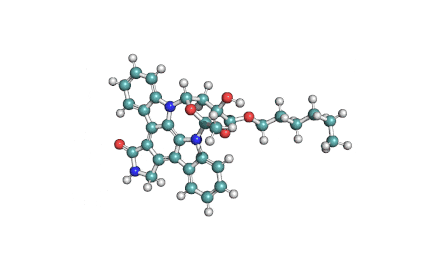
KT5720: A selective inhibitor of cAMP-dependent protein kinase (PKA)
Further Information
Soluble in methanol, DMSO
indolocarbazole alkaloid
PKA inhibitor
Composition
Special Info
Other Fields



Antibiotics are antimicrobial compounds used in the treatment and prevention of bacterial infection. They can either kill or they can inhibit the growth of bacteria. Several antibiotics are also effective against fungi and protozoans. Some are toxic to humans and animals, even when given in therapeutic dosages. Antibacterial antibiotics are commonly classified based on their mechanism of action, chemical structure, or spectrum of activity. Most antibiotics target bacterial metabolic functions or growth processes. Those antibiotics that interfere with the function of essential bacterial enzymes, or that target the bacterial cell wall or the cell membrane, are usually bactericidal. Those that target protein synthesis are usually bacteriostatic. Further categorizations can be made based on target specificity. Use of antibiotics is essential in retaining the purity of particular selection factors in populations of cells. In recent years antibiotics have been found to be valuable in a wide range of life science disciplines. Fermentek has had particular success in manufacturing antibiotics using fermentation processes.
KT5720: A selective inhibitor of cAMP-dependent protein kinase (PKA)
Soluble in methanol, DMSO
indolocarbazole alkaloid
PKA inhibitor




Synonyms:
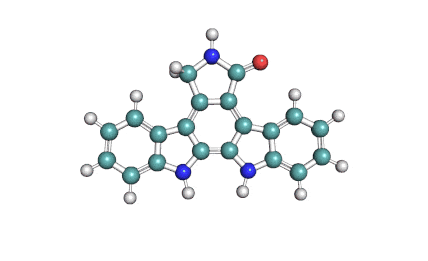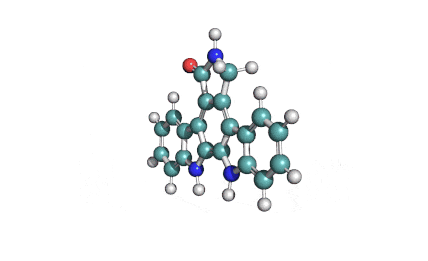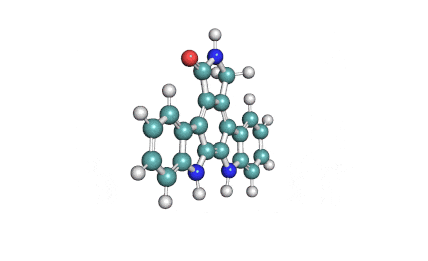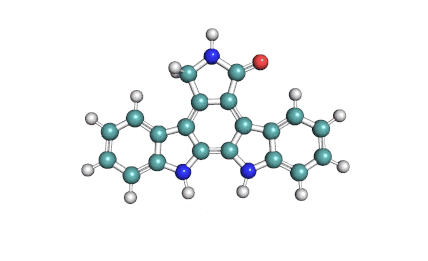
K252c: Cell permeable protein kinase inhibitor of the Staurosporine family (indolo[2,3-a]carbazole alkaloids)
Readily soluble in chloroform, acetonitrile, acetone, dioxane, tetrahydrofuran, pyridine;Soluble in ethanol, methanol, 1-propanol, ethyl acetate and n-butanol;Insoluble in water, 2-propanol. Comment: This information has been collected from available scientific sources. It is not a part of product specification
indolocarbazole alkaloidPKC inhibitor
K252c inhibits protein kinase C. Its reported IC50 value of 214 nM on rat brain enzyme K252c is cytotoxic for A549 and P388 cancer celllines showing IC50 = 2-3 μM


Synonyms:
K252B: an inhibitor of protein kinase A, C, G
DMSO or Methanol
Indolocarbazole alkaloid;
PK inhibitor
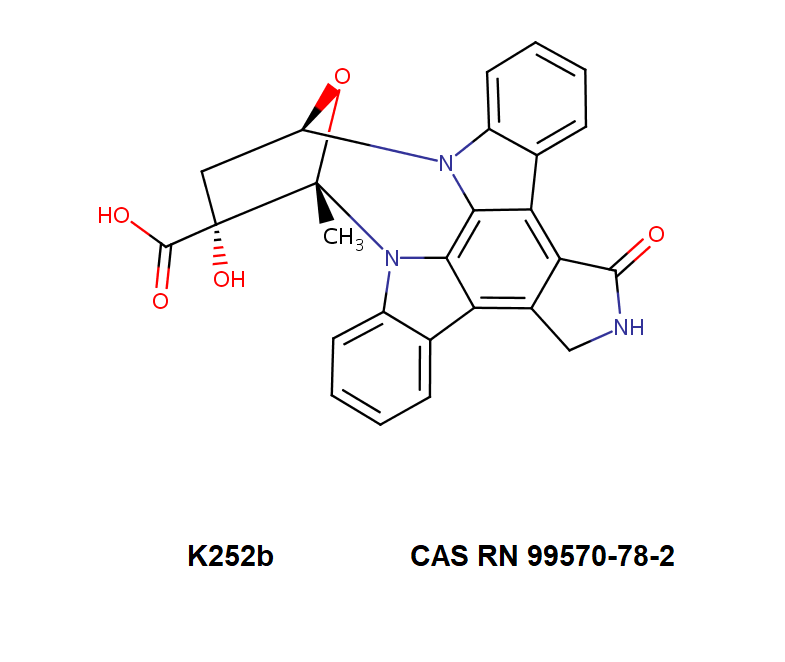


RTECS NZ0550000
K252a: Cell permeable protein kinase inhibitor
Readily soluble in chloroform, acetonitrile, acetone, dioxane, tetrahydrofuran, pyridine;soluble in ethanol, methanol, 1-propanol, ethyl acetate and n-butanol; insoluble in water, 2-propanol
K252a is a highly potent cell permeable inhibitor of CaM kinase and phosphorylase kinase . At high concentrations it also inhibitors of serine/threonine protein kinases. K252a inhibits tyrosine phosphorylation of Trk A induced by NGF K252a is reported to promote myogenic differentiation in C2 mouse myoblasts




(4R,6S,8S,10Z,12R,14R,16E,18S,19S,20R,21S)-11,19,21-Trihydroxy-22-{(2S,2'S,5S,5'R)-5'-[(1S)-1-hydroxyethyl]-2,5'-dimethyloctahydro-2,2'-bifuran-5-yl}-4,6,8,12,14,18,20-heptamethyl-9-oxo-10,16-docosadi enoic acid
RTECS : NO0600000
Ionomycin : a Ca++ ionophore
DMSO, Ethanol, Methanol
Ionomycin is more effective than A23187 as a Ca++ionophore. Ionomycin is used in research on Ca++ transport across biological membranes; Ionomycin induces apoptotic degeneration of embryonic cortical neuron
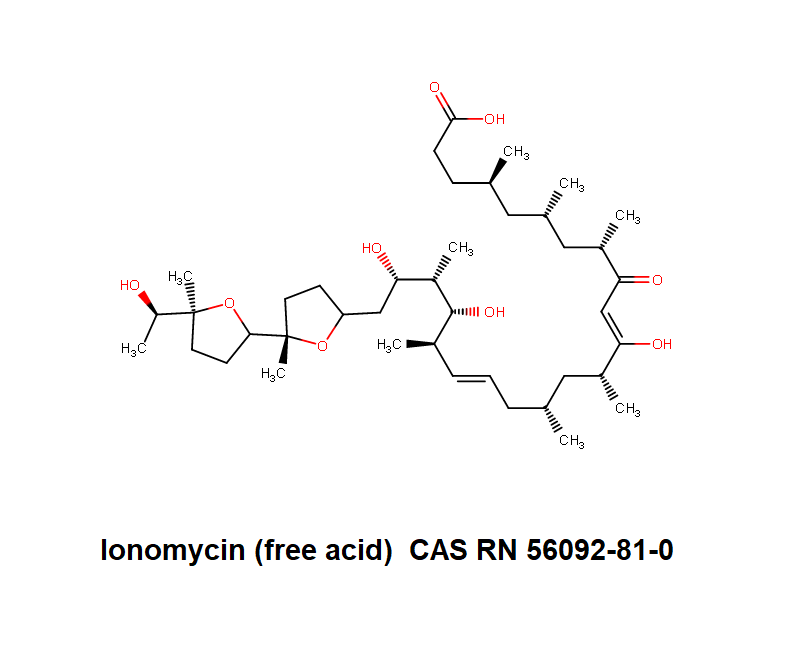


RTECS NO0650000
Ionomycin : a potent and selective Ca++ ionophore
DMSO, Ethanol, Methanol
Ionomycin is more effective than A23187 as a Ca++ionophore. Ionomycin is used in research on Ca++ transport across biological membranes; Ionomycin induces apoptotic degeneration of embryonic cortical neurons.


Genistein: an isoflavone with anticancer, antiproliferation, and chemopreventive effects. Genistein induces cell differentiation. Genistein inhibits protein histidine kinase.
Genistein is soluble in DMSO, Ethanol.
isoflavonoid
histidine kinase inhibitor


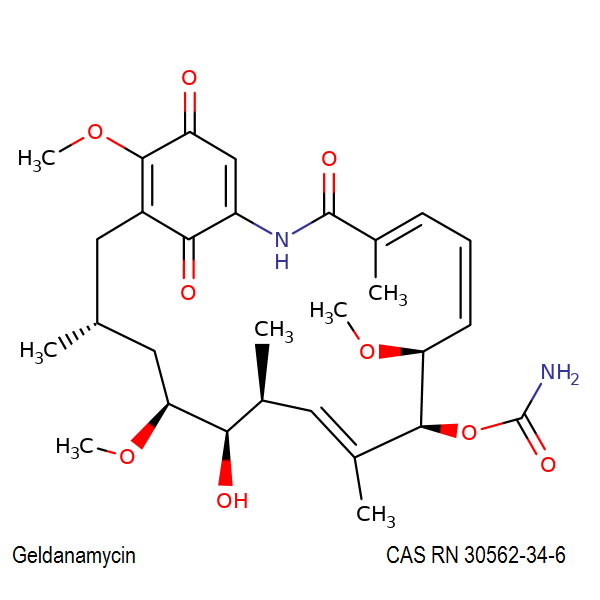
RTECS LX8920000
IUPAC Name : [(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-6-hydroxy-5,11,21-trimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate I
Geldanamycin is a benzoquinone ansamycin antibiotic, which binds to Hsp90 (Heat Shock Protein 90) and alters its function. Geldanamycin is a natural product from which several valuable derivates were synthesized. All derivates share its HSP90 inhibition, and vary in overall toxicity and water solubility.
Geldanamycin is soluble in DMSO, Dichloromethane. Geldanamycin is insoluble in water.
Benzoquinone ansamycin antibiotic ;
HSP90 inhibitor ;
Tyrosine Kinase Inhibitor ;
Exhibits potent antitumor activity. It also inhibits nuclear hormone receptors.


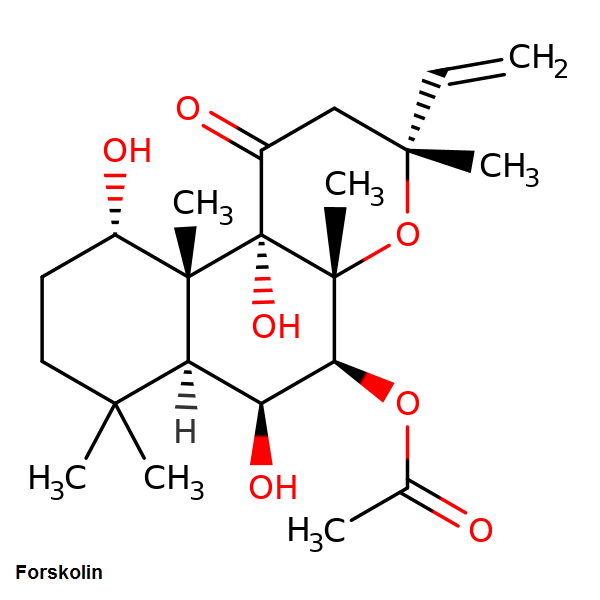
Forskolin is a labdane diterpene that is produced by Coleus forskohlii
Forskolin is soluble in DMSO, Methanol, Ethanol, Dichloromethane, Forskolin may be dissolved in 2% ethanol in water, by dissolving it first in Ethanol and subsequently diluting this solution with water.


Synonyms: FK506, Tacrolimus,
RTECS: KD4200000
FK506 is 23-membered macrolide lactone discovered in 1984, by Kino T, Hatanaka H and Hashimoto M. [reference 2] FK506 exhibits potent inhibitory effect on T- lymphocyte activation. It binds to immunophilins FK506 binding proteins (FKBP-12) and a complex of FKBP-12, calcium, calmodulin and calcineurin is formed, inhibiting phosphatase activity of calcineurin. This prevents dephosphorylation and translocation of nuclear factor of activated T – cells (NF-AT) and inhibits transcription of early T-cell activation gene, Interleukin-2, Tumour Necrosis Factor (TNF-a) and proto-oncogenes; suppressing expression of IL-2 and IL-7 receptor. This results in inhibition of T-lymphocyte activation. FK506 also inhibits the mixed lymphocyte reaction, generation of cytotoxic T-cells and T-cell dependent B-cell activation.
FK506 is soluble in Methanol, Ethanol, Acetone, Ethyl Acetate, Diethyl ether, Chloroform, Dicloromethane. FK506 is sparingly soluble in Hexane, Petroleum ether. FK506 is insoluble in water
Chemical classification:
Macrolide
Bioactivity classification
Immunosuppressant
FK506 (Tacrolimus) is used as an immunosuppressant at organ transplantations. For experimental uses of FK506 (Tacrolimus) read also United States Patent Application 20090098200