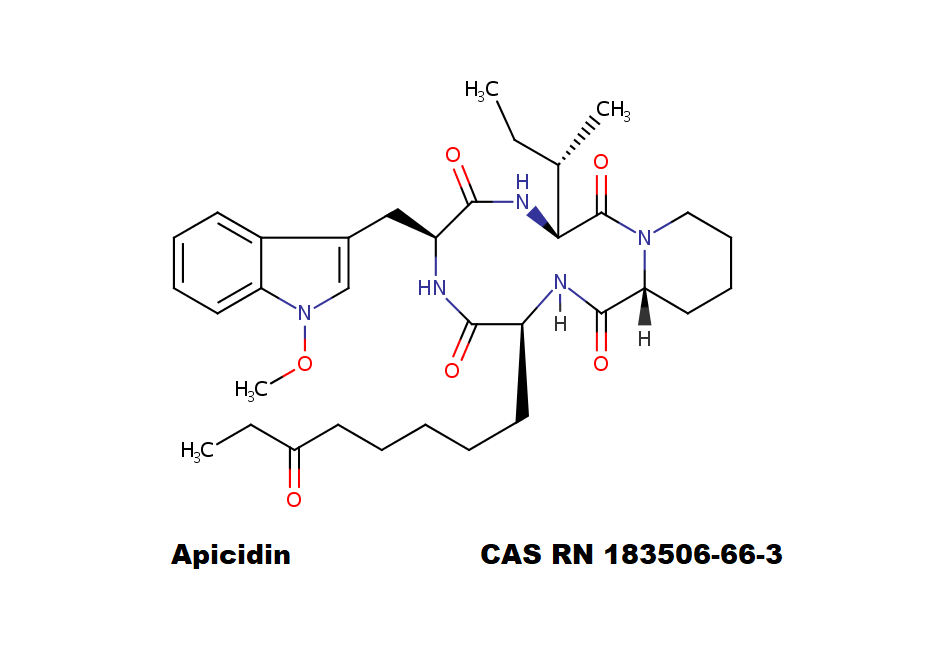
Apicidin
Details
Specifications
Chemical identification
Synonyms:
- apicidin C
IUPAC name:
- (3S,6S,9S,12R)-3-[(2S)-butan-2-yl]-6-[(1-methoxyindol-3-yl)methyl]-9-(6-oxooctyl)-1,4,7,10-tetrazabicyclo[10.4.0]hexadecane-2,5,8,11-tetrone
RTECS XJ6094046
Apicidin is a mycotoxin which may occur naturally, as a result of Fusarium fungi growing on improperly stored foods.
Apicidin is a potent cell permeable inhibitor of histone deacetylase.
Further Information
Ethanol, DMSO, Dichloromethane. Slightly soluble in water, methanol.
- Cyclic peptide antibiotic
- Mycotoxin
- Antiprotozoal
- HDAC inhibitor
- Angiogenesis inhibitor
Cyclic peptide antibiotic with broad spectrum antiparasitic and antiprotozoan activity. An histone deacetylase inhibitor, Anti-angiogenic; Apoptosis inductor.
Composition
Special Info
Other Fields