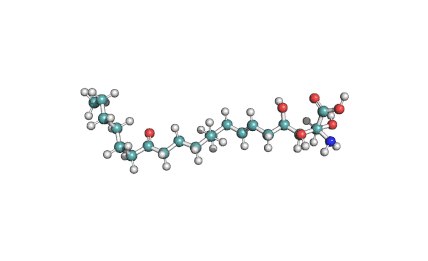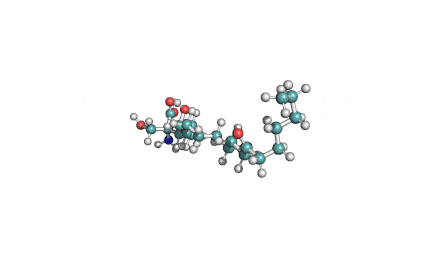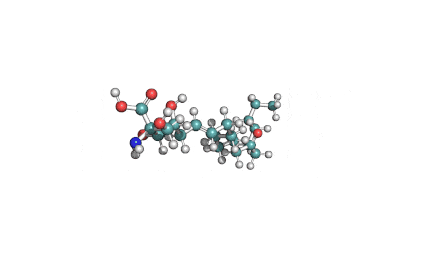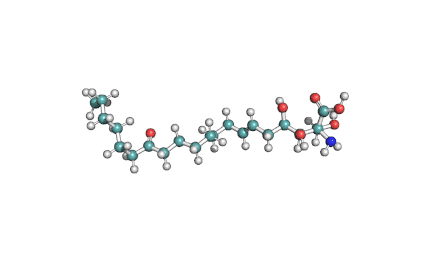
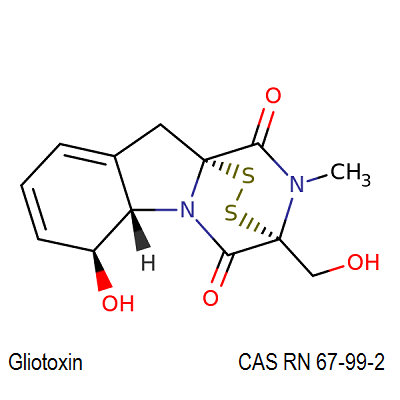
Myriocin
Details
Specifications
Clear colorless solution at 5mg/ml of DMSO (slight heating)
Chemical identification
- Myriocin
- Thermozymocidin,
- ISP-I
- (2S,3R,4R,6E)-2-Amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-6-eicosenoic acid
Fungal antibiotic, atypic amino acid, immunomodulator, serine palmitoyltransferase inhibitor, apoptosis inducer.
Further Information
Myriocin is slightly soluble in DMSO, alcohols.Myriocin is insoluble in water and in most organic solvents.
- amino-acid antibiotic
- immunosupressor
- serine palmitoyltransferase inhibitor
Myriocin, a potent inhibitor of serine palmitoyltransferase,a (the 1st step in sphingosine biosynthesis), is used in biochemical research as a tool for depleting cells of sphingolipids. Myriocin is a potent immunosuppressor, reportedly 10 to 100 times stronger than cyclosporin.
Composition
Supply related information
Special Info
Other Fields