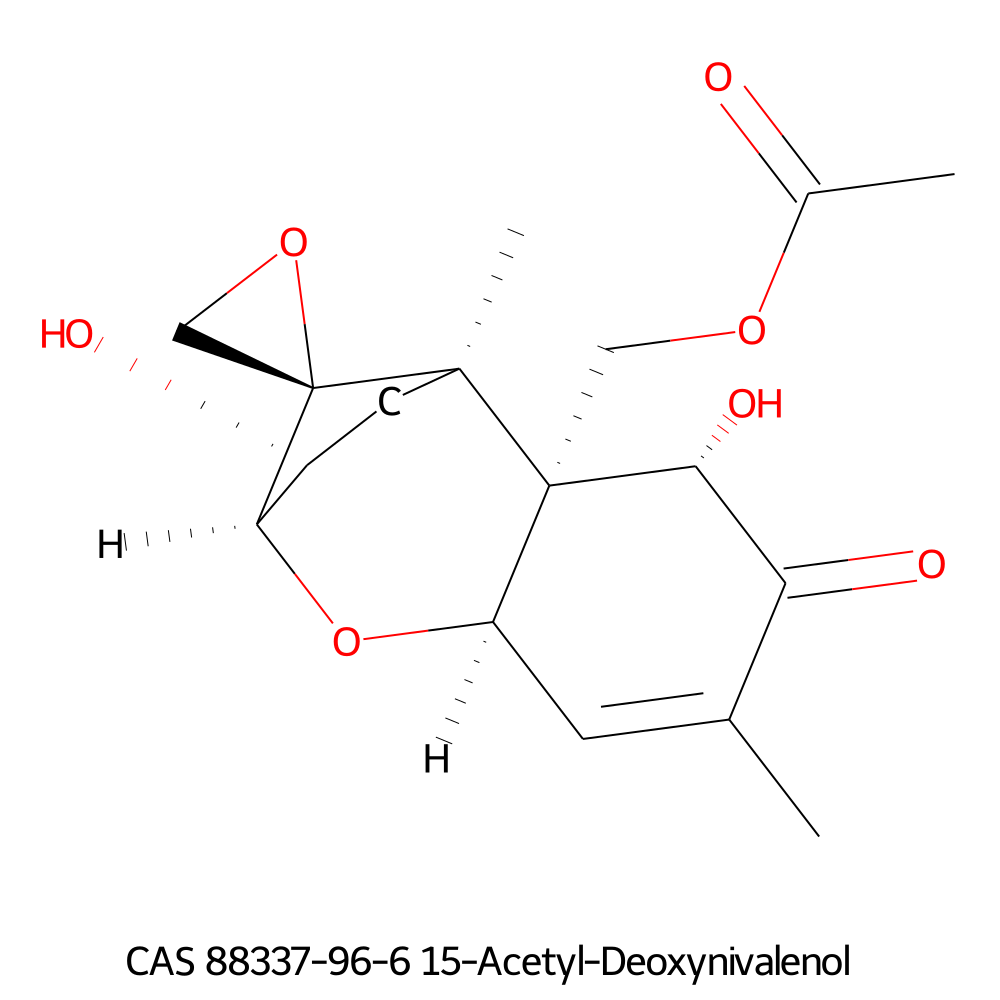
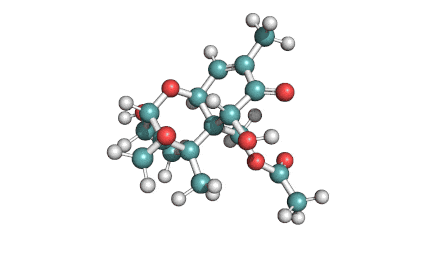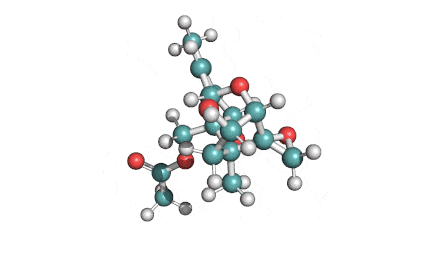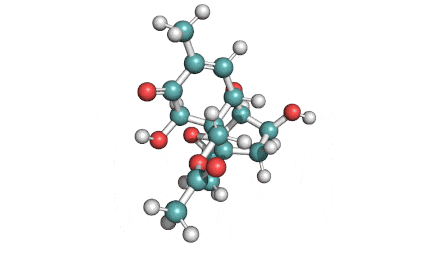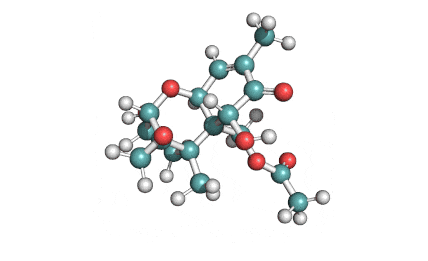
15 Acetyl Deoxynivalenol
Details
Specifications
Chemical identification
Natural, Type B trichothecene mycotoxin
Further Information
15-acetyl Deoxynivalenol is soluble in common polar organic solvents as acetonitrile, methanol and ethyl acetate, only slightly soluble in water. Trichothecenes with acetoxy-groups are unstable in methanol, even when stored in a freezer [H. Pettersson, EU-Commission Progress Report SMT4-CT96-2047, 1-24, (1998), "Intercomparison of trichothecene analysis and feasibility to produce certified calibrants and reference material"] Acetonitrile is recommended as solvent for 15-acetyl Deoxynivalenol as it has no significant UV absorbance below 220 nm.
Trichothecene mycotoxin
Composition
Special Info
Other Fields