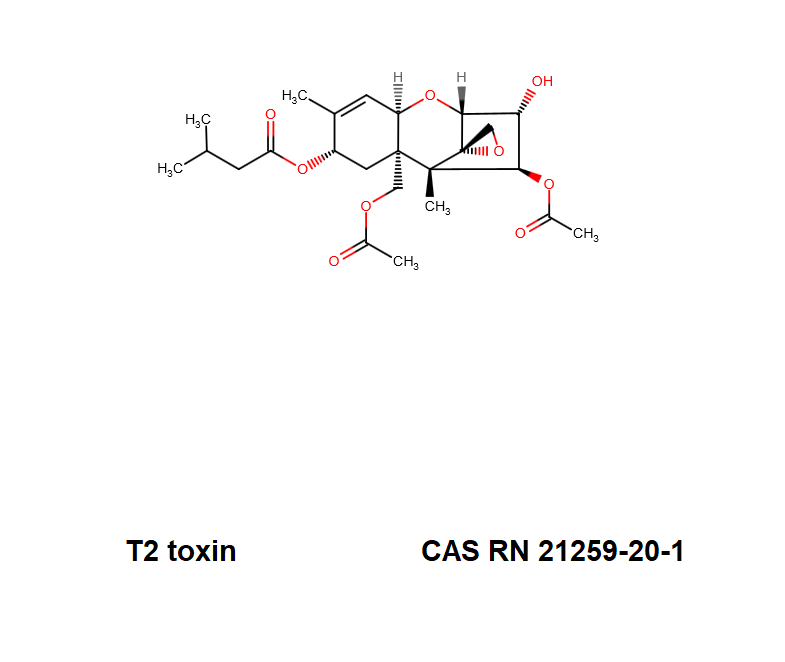
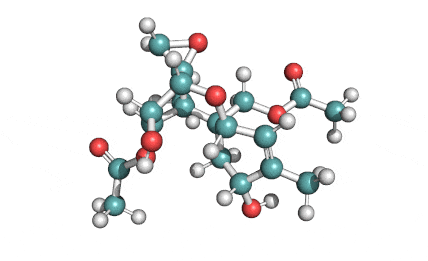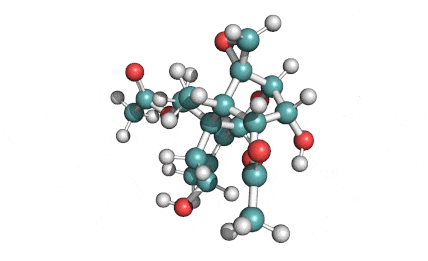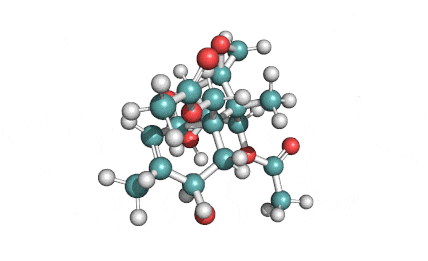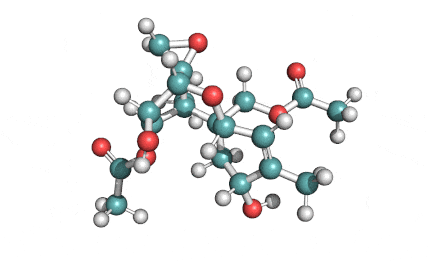
T2 Toxin
Details
Specifications
Chemical identification
Synonyms
- Fusariotoxin T2
- T2 TOXIN
- MYCOTOXIN T2
- 8-(3-Methylbutyryloxy)-diacetoxyscirpenol
- Trichothec-9-ene-3.alpha.,8.alpha.,15-tetrol, 12,13-epoxy-, 4,15-diacetate 8-isovalerate
RTECS: YD0100000
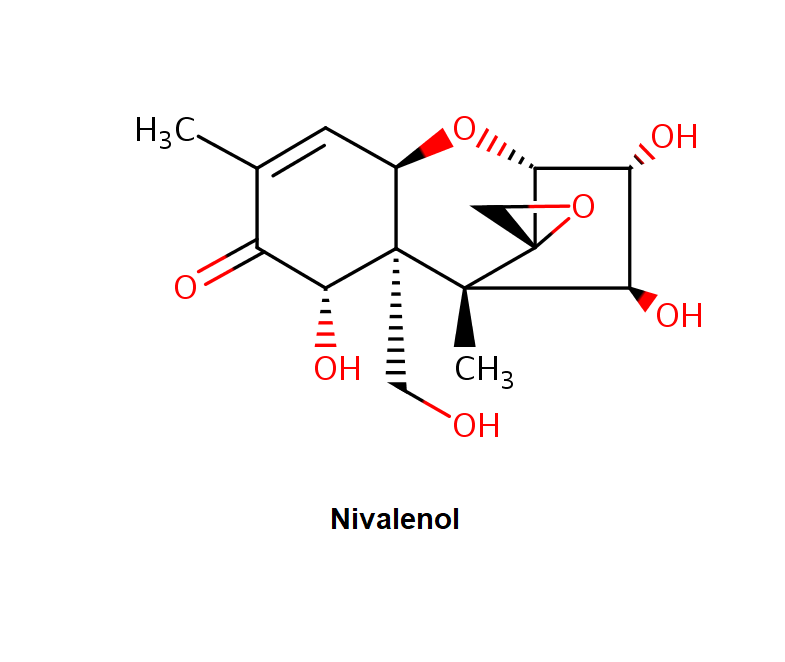
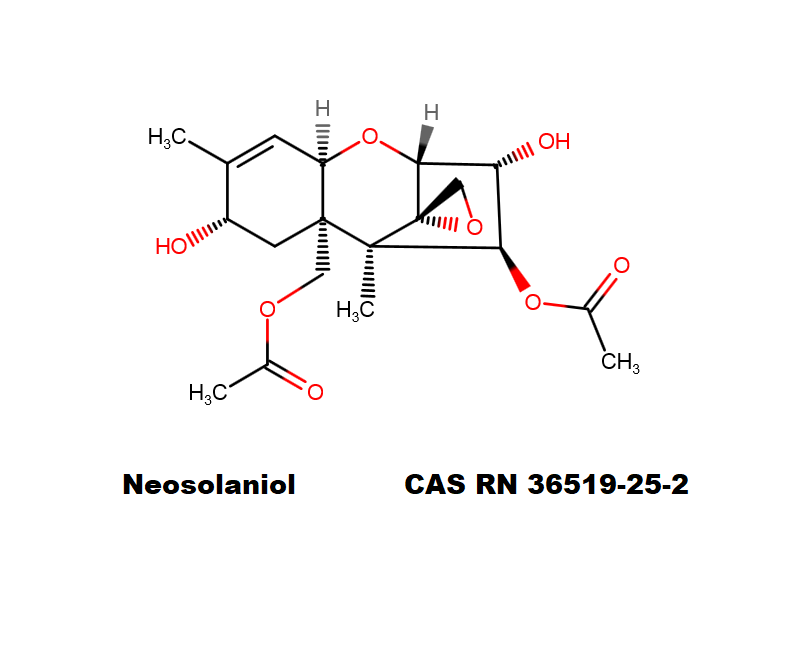
T2 Toxin: A trichothecene group mycotoxin, of the most important Fusarium toxins.
Further Information
Dichloromethane, DMSO, Ethanol, Ethyl Acetate. Slightly soluble in petroleum ether; very slightly soluble in water.
Trichothecene Mycotoxin
T-2 toxin has been reported to be used to increase blood-brain barrier permeability in rats.
T-2 Toxin induces DNA damage and cell death on prolonged administration.
Composition
Special Info
Other Fields