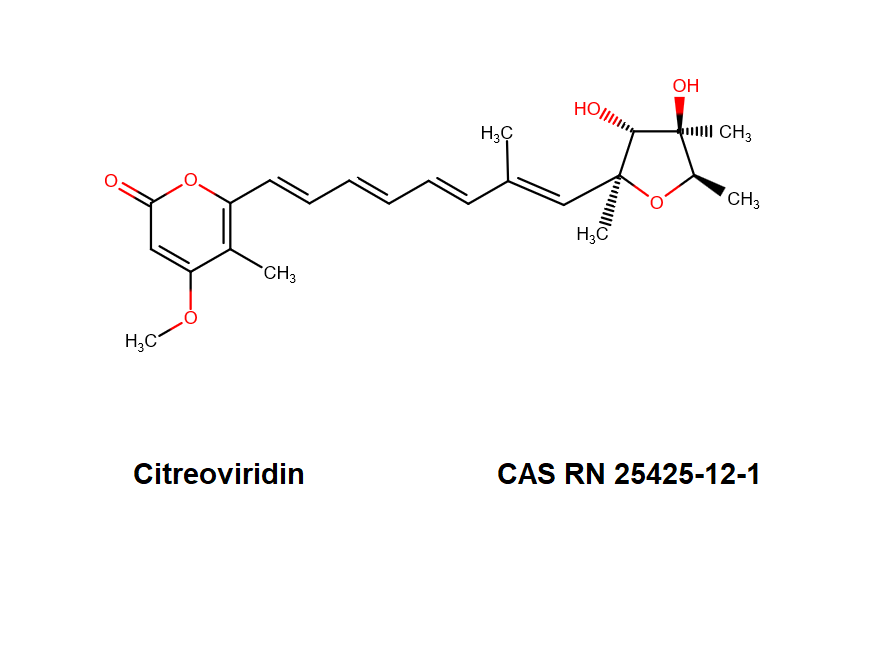
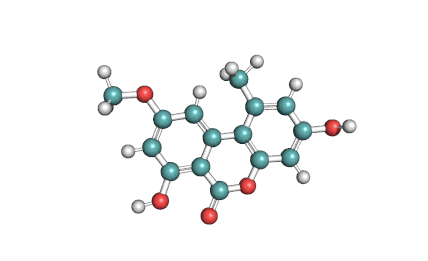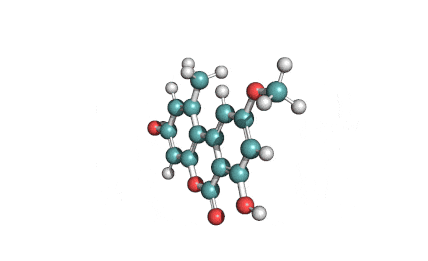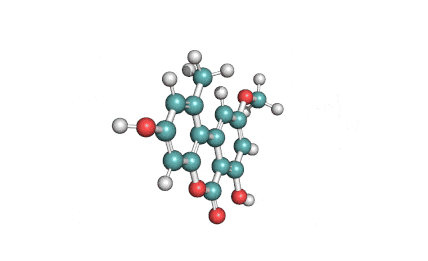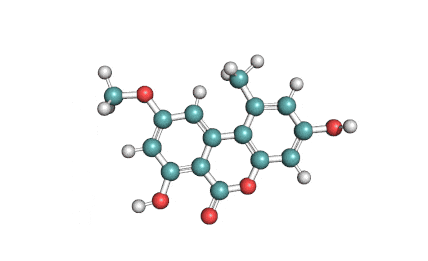
Citreoviridin
Details
Specifications
Chemical identification
Synonyms
- Citreoviridin A
- 4-Methoxy-5-methyl-6-(7-methyl-8-(tetrahydro-3,4-dihydroxy-2,4,5-trimethyl-2-furyl)-1,3,5,7-octatetraenyl)-2H-pyran-2-one
- IUPAC Name: 6-[(1E,3E,5E,7E)-8-[(2S,3R,4R,5R)-3,4-Dihydroxy-2,4,5-trimethyloxolan-2-yl]-7-methylocta-1,3,5,7-tetraenyl]-4-methoxy-5-methylpyran-2-one
RTECS: UQ1235000
Citreoviridin is a neurotoxic mycotoxin from Penicillium citeoviride, Penicillium toxicarium, Penicillium ochrosalmoneum, Penicillium ochrosalmoneum, Aspergillus terreus, and several other related fungi. Citreoviridin is believed to be the cause of the Acute Cardiac Beri-Beri disease. Naturally, It is formed on rice improperly stored.
Further Information
Citreoviridin is soluble in benzene, ethanol, chloroform, ether, dichloromethane. Citreoviridin is hardly soluble in water (enough to give it yellow color) and hexane
Aurovertin class mycotoxin
Comment: Aurovertins are very toxic and complex pyrone derivatives of fungal origin . They bind to and inhibit mitochondrial ATPase, thereby uncoupling oxidative phosphorylation. They are used as biochemical tools.
As other Aurovertin group members, Citreoviridin binds to and inhibit mitochondrial ATPase, thereby uncoupling oxidative phosphorylation.
Citreoviridin, a mycotoxin that has been shown to inhibit the mitochondrial ATP synthetase system. It inhibits soluble ATPase, ADP-stimulated respiration in isolated rat liver mitochondria, and ATP-driven reduction of NAD+ by succinate (KD = 2 µM). Citreoviridin has been used to target ectopic ATPase activity in cancer cells in order to modulate the metabolic activity associated with tumorigenesis.
Composition
Special Info
Other Fields