Leptomycin B
Details
Specifications
Chemical identification
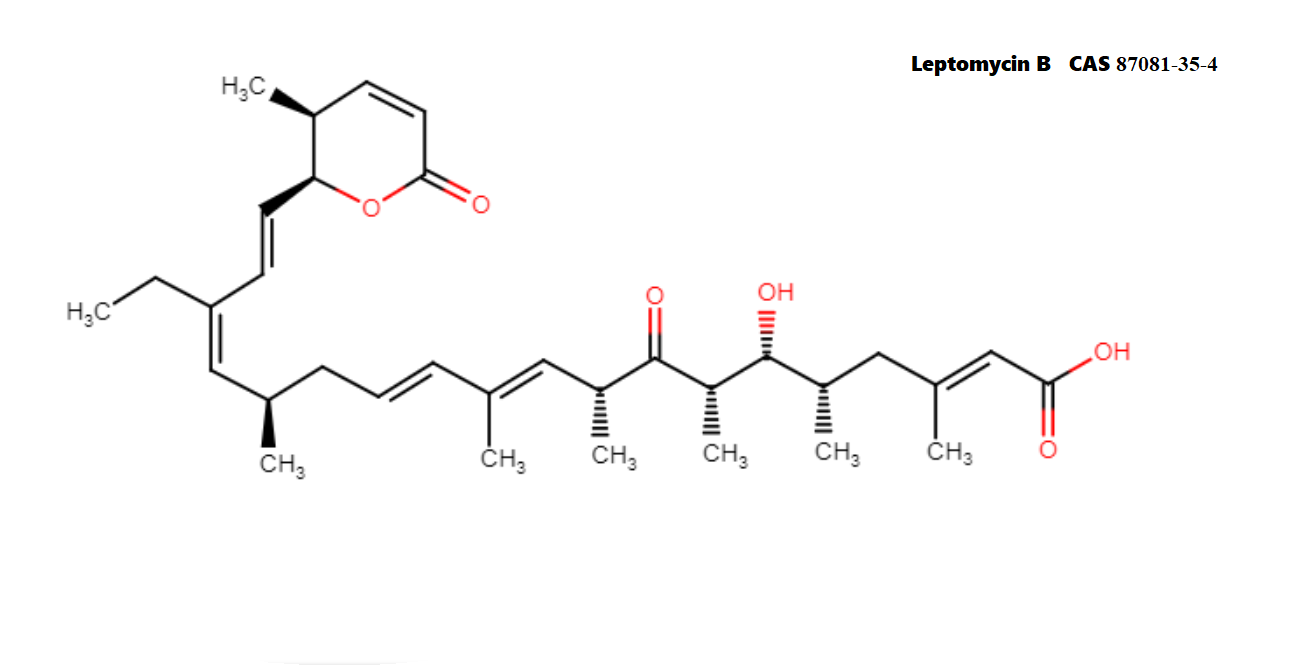
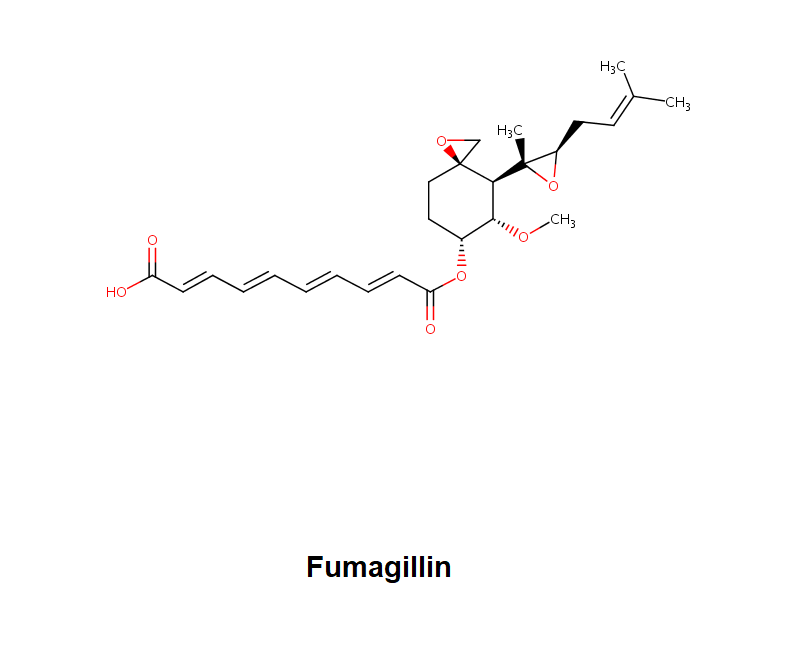
Leptomycin B is an unsaturated, branched-chain fatty acid.
Until 4-jun-2018 Leptomycin had been offered by Fermentek in the form of 1% V/W solution in Methanol/Water mix. Since then, it is offered as lyophylizate.
Further Information
Leptomycin B is soluble in ethanol, methanol.
Until 4-jun-2018, Fermentek supplied Leptomycin in form of 1% w/v solution in Methanol/water. Since 4-jun-2018 it is as a lyophylisate.
Fatty acid antibiotic
Leptomycin B is an important tool in the study of nuclear export. Leptomycin B is twice as potent as Leptomycin A Leptomycin B (LPB) was originally discovered as a potent anti-fungal antibiotic from Streptomyces sp. However, recent data (2003) showed that Leptomycin causes G1 cell cycle arrest in mammalian cells and is a potent anti-tumor agent against murine experimental tumors. Leptomycin B is a potent and specific nuclear export inhibitor. Leptomycin B alkylates and inhibits CRM1 (chromosomal region maintenance)/exportin 1, a protein required for nuclear export of proteins containing a nuclear export sequence (NES). In addition to antifungal and antibacterial activities, Leptomycin B blocks the cell cycle and is a potent anti-tumor agent. At low nM concentrations, Leptomycin B blocks the nuclear export of many proteins including HIV-1 Rev, MAPK/ERK, and NF-κB/IκB, and it stabilizes the expression of p53. Leptomycin B also inhibits the export and translation of many RNAs, including COX-2 and c-Fos mRNAs, by inhibiting export of ribonucleoproteins.
Composition
Special Info
Other Fields