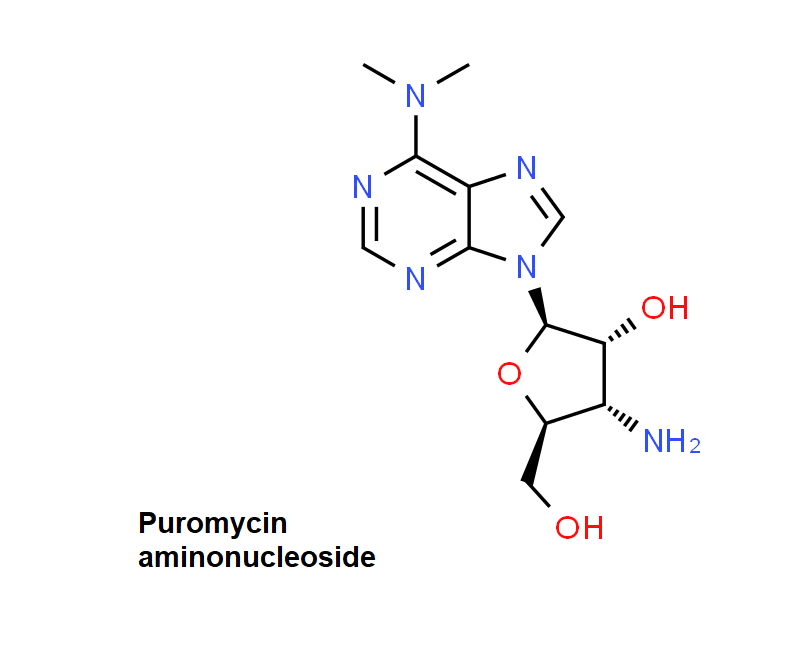
Puromycin Aminonucleoside
Details
Specifications
Clear colorless solution at 10 mg/ml water
Chemical identification
RTECS: AU7337000
Puromycin Aminonucleoside is a puromycin analog which does not inhibit protein synthesis or induce apoptosis
Further Information
Water
- Nucleoside antibiotic
- Protein synthesis inhibitor
Composition
Special Info
Other Fields