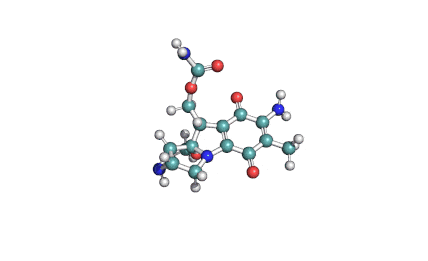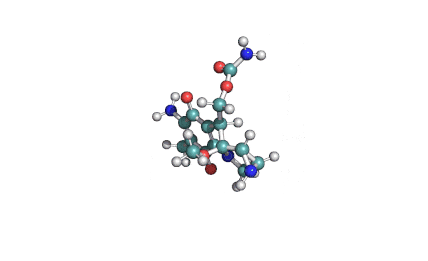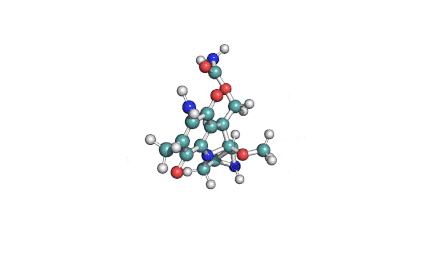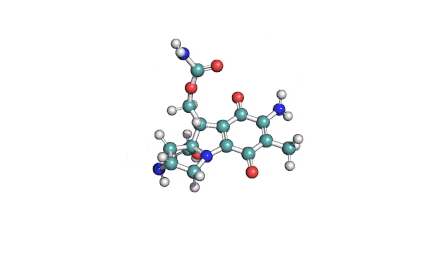
Fumitremorgin C
Details
Specifications
Methanol or Acetonitrile.
Chemical identification
Synonyms:
Chemical names:
IUPAC: (5aS,12S,14aS)-9-Methoxy-12-(2-methyl-1-propen-1-yl)-1,2,3,5a,6,11,12,14a-octahydro-5H,14H-pyrrolo[1'',2'':4',5']pyrazino[1',2':1,6]pyrido[3,4-b]indole-5,14-dione
RTECS# UY8709670
Fumitremorgin C is a tremorgenic mycotoxin isolated from Aspergillus fumigatus. Recent research has recognized Fumitremorgin C as a potent and specific inhibitor of the breast cancer resistance protein (BCRP/ABCG2) multidrug transporter. It reverses multidrug resistance mediated by BCRP and increases cytotoxicity of several anticancer agents in vitro.
Further Information
Soluble in Ethanol, Methanol, DMF, DMSO Chloroform.
Negligible solubility ( 35 mg in 1 liter water @25°C) has been reported.
Breast cancer resistance protein inhibitor
Mammalian cell cycle inhibitors
Composition
Special Info
Other Fields