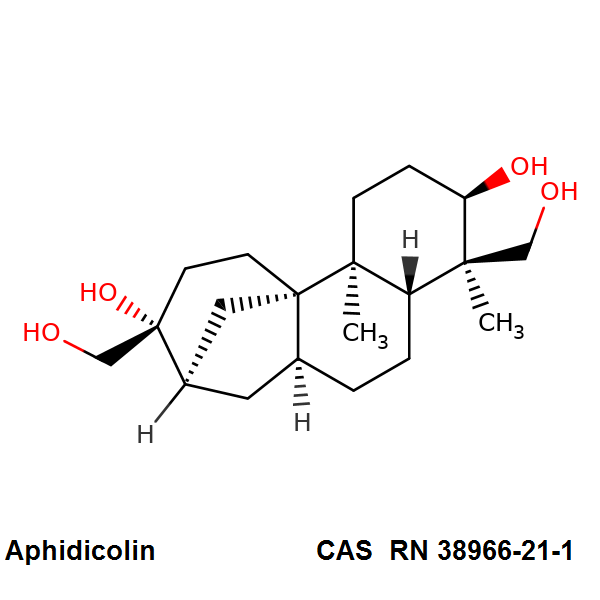
Aphidicolin
Details
Specifications
Chemical identification
RTECS: PB9185000
Aphidicolin is an antiviral antibiotic produced by Nigrospora oryzae and other fungi. Aphidicolin inhibits the growth of eukaryotic cells and certain animal viruses by selectively inhibiting the cellular replication of DNA polymerase II or the viral-induced DNA polymerases. The drug may be useful for controlling excessive cell proliferation in patients with cancer, psoriasis or other dermatitis, while having no effect on non-multiplying cells.
Further Information
Methanol, DMSO
tetracyclic mycotoxin
DNA polymerase A inhibitor
Antiviral
Chemical classification:
- A tetracyclic diterpene antibiotic.
Biological classification:
- Antiviral, antimithotical
- Specific inhibitor of DNA polymerase A
Aphidicolin inhibits the growth of eukaryotic cells and certain animal viruses by selectively inhibiting the cellular replication of DNA polymerase II or the viral-induced DNA polymerases. The drug may be useful for controlling excessive cell proliferation in patients with cancer, psoriasis or other dermatitis with little or no adverse effect upon non-multiplying cells.Aphidicolin is a tool in cell proliferation and differentiation research. Aphidicolin may possibly be used for controlling excessive cell proliferation in cancer, psoriasis or other dermatitis with little or no adverse effect upon non-multiplying cells.
Composition
Special Info
Other Fields