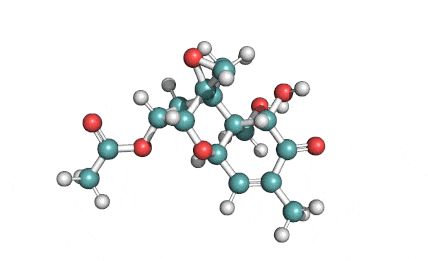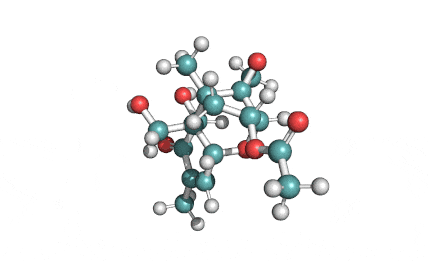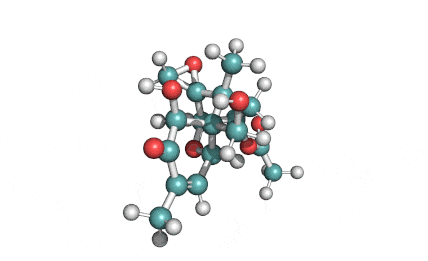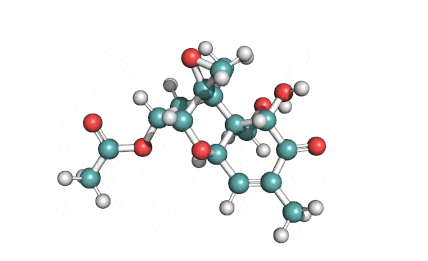
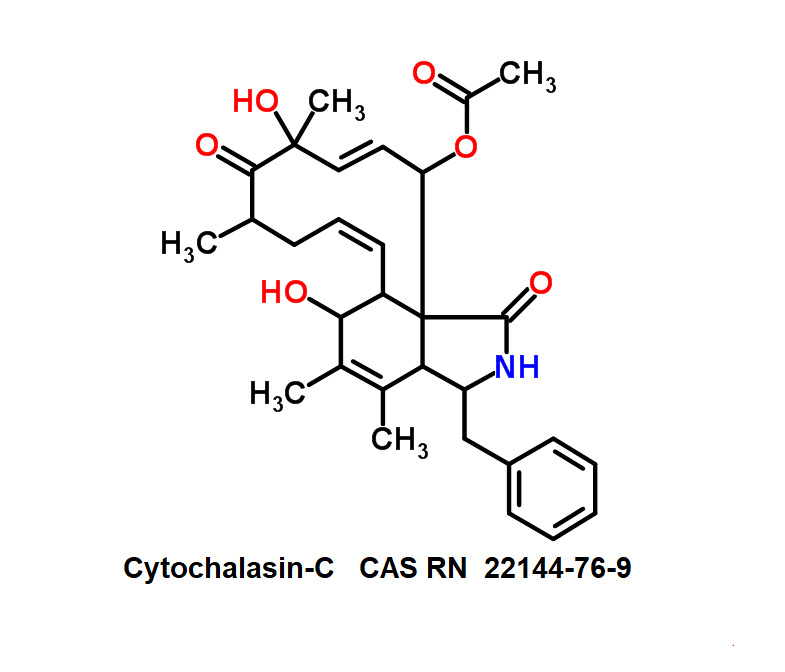
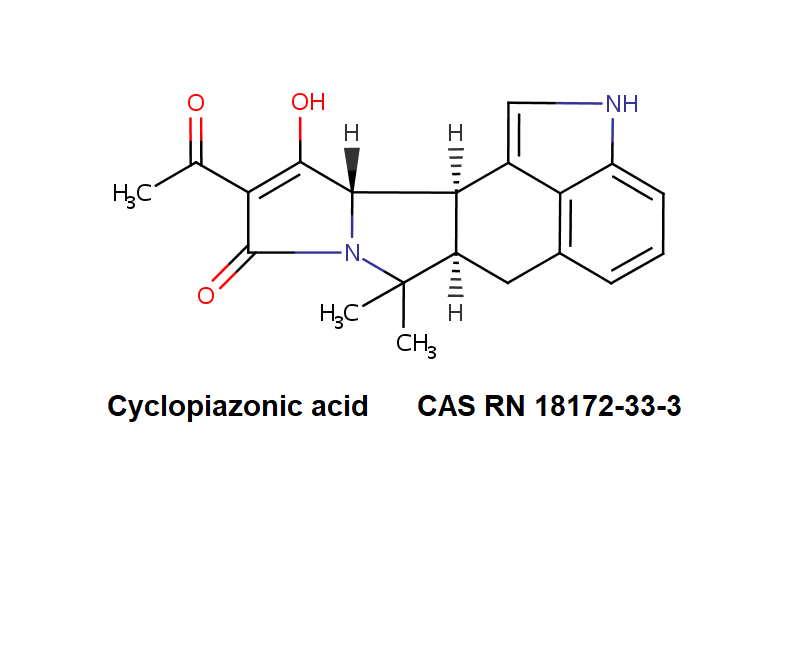
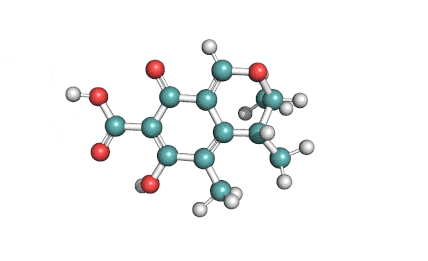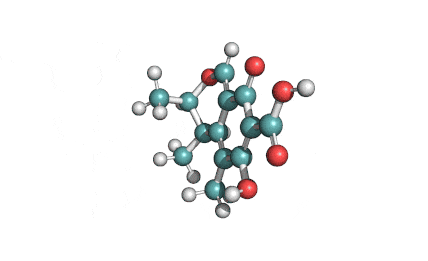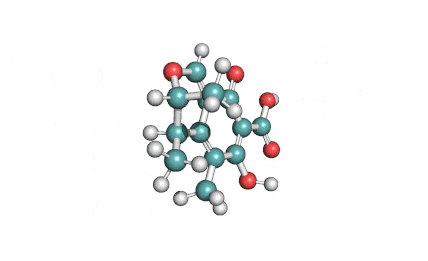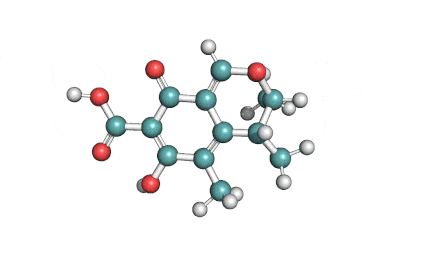
3 Acetyl Deoxynivalenol
Details
Specifications
Chemical identification
- 3ADON
- 3α-Acetylvomitoxin
- 3-Acetyl DON
- 3-Acetyldeoxynivalenol
- Dehydronivalenol monoacetate
- Deoxynivalenol monoacetate
RTECS: YD0167000
Natural Type B trichothecene mycotoxin
Further Information
3-acetyl Deoxynivalenol is soluble in common polar organic solvents as acetonitrile, methanol and ethyl acetate, only slightly soluble in water.
Trichothecene mycotoxin
Composition
Special Info
Other Fields