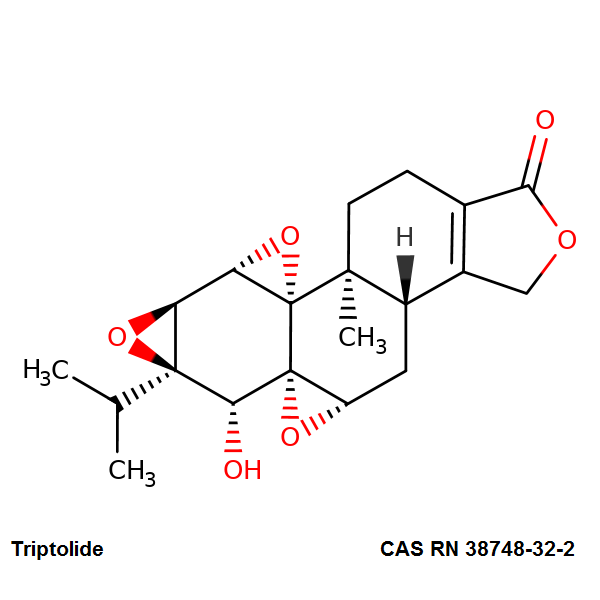
Triptolide
Details
Specifications
Chemical identification
Synonyms:
- NSC 163062
- PG490
Chemical names:
Trisoxireno[4b,5:6,7:8a,9]phenanthro[1,2-c]furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-
IUPAC:
(1S,2S,4S,5S,7R,8R,9S,11S,13S)-8-hydroxy-1-methyl-7-(propan-2-yl)-3,6,10,16-tetraoxaheptacyclo[11.7.0.0^{2,4}.0^{2,9}.0^{5,7}.0^{9,11}.0^{14,18}]icos-14(18)-en-17-one
RTECS#: YK7751000
Triptolide is a diterpenoid epoxide which is produced by Tripterygium wilfordii, a vine used in traditional Chinese medicine. Triptolide has pharmacological activities including anti-inflammatory, immune-supressing, antiproliferative anti-angiogenetic, and proapoptotic activity. It has in vitro and in vivo activities against mouse models of polycystic kidney disease and pancreatic cancer.
Further Information
Reportedly: Insoluble in water. Insoluble in Ethanol. Soluble up to 0.2Molar ( 72 mg/ml) of DMSO
Chemical classification: Terpenoid
Composition
Supply related information
Special Info
Other Fields