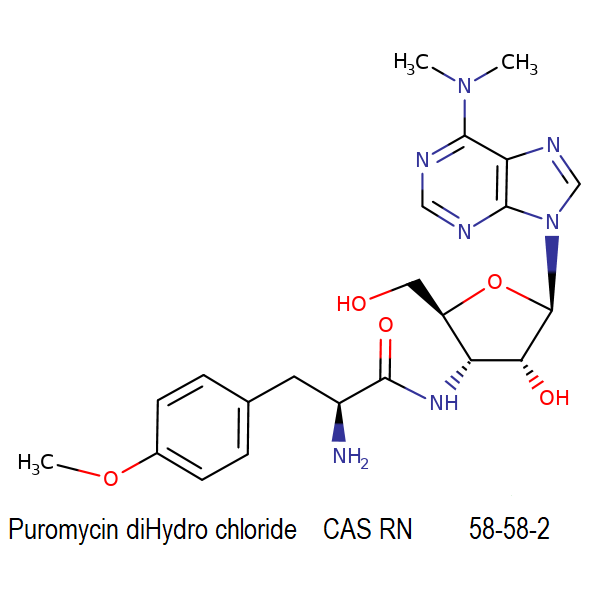
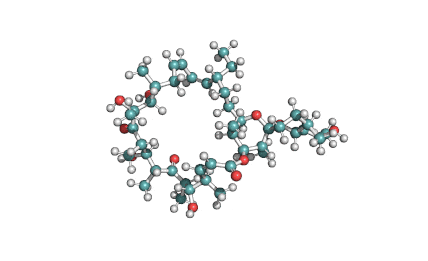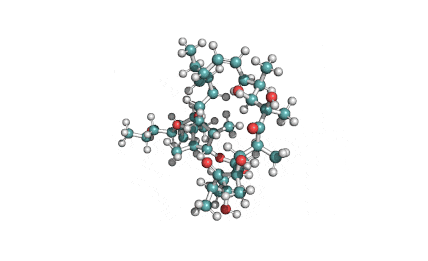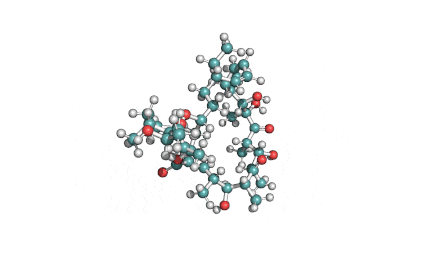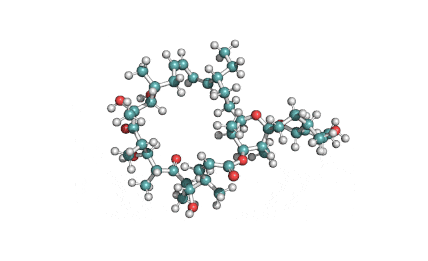
Valinomycin
Details
Specifications
Chemical identification
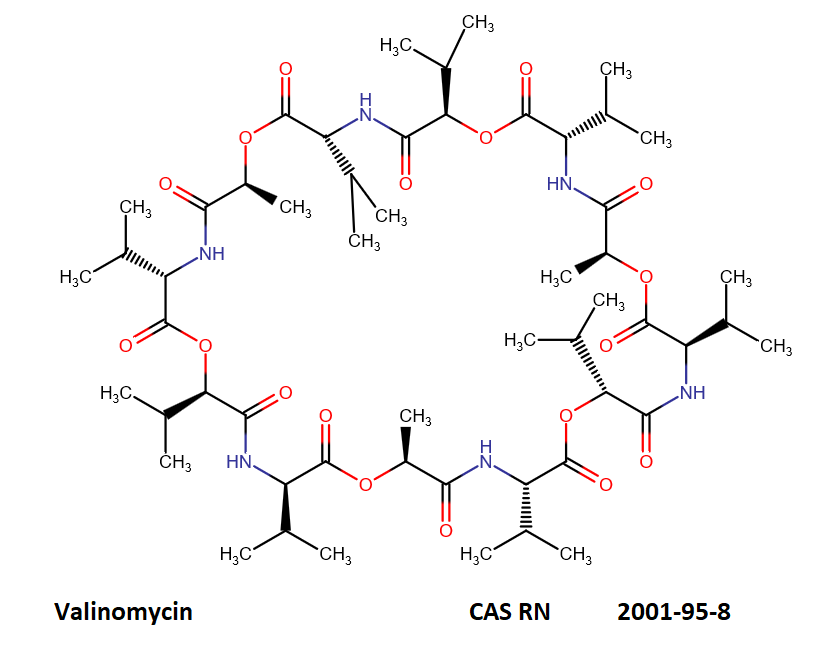
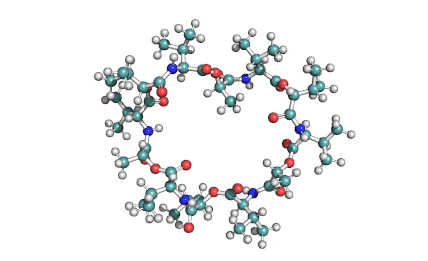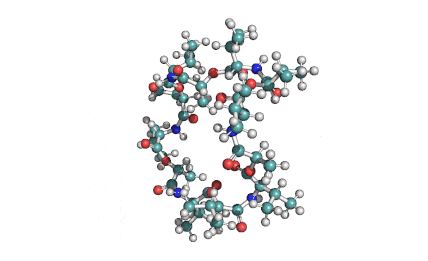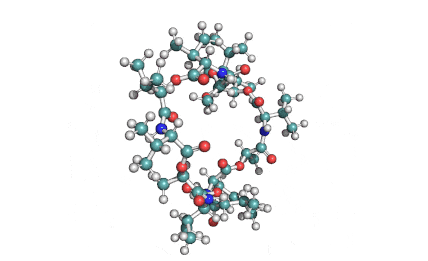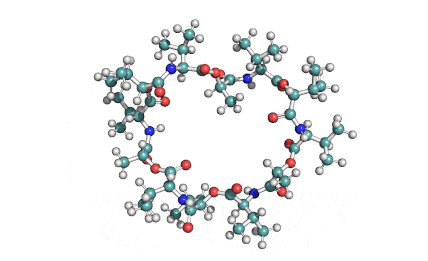
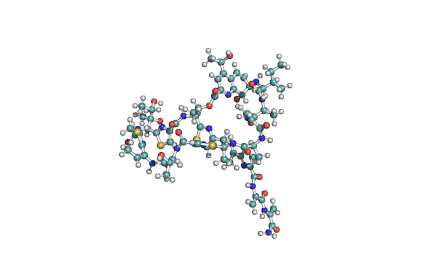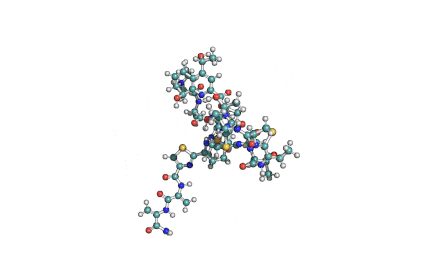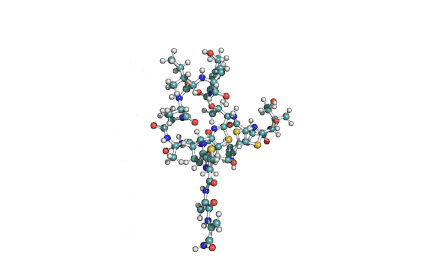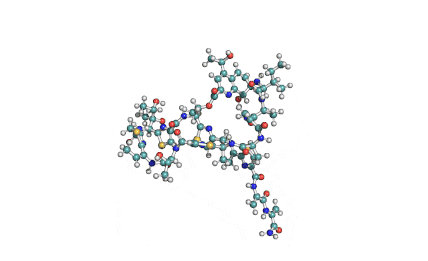
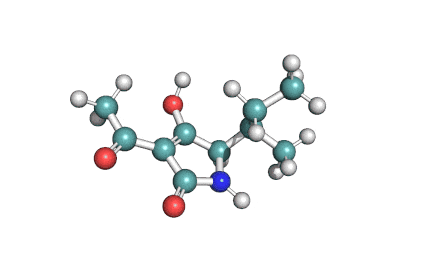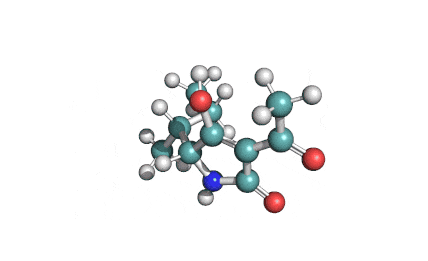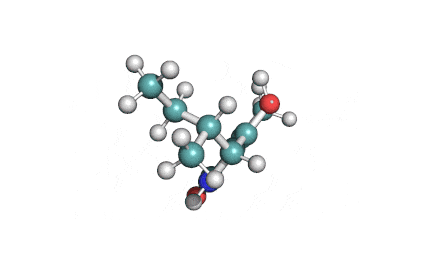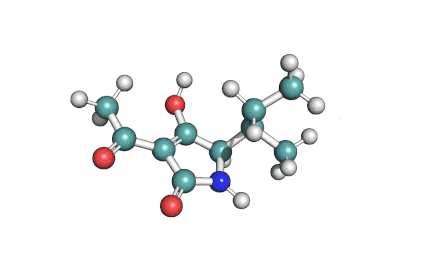
Valinomycin is a cyclododecadepsipeptide ionophore antibiotic produced by Streptomyces fulvissimus and chemically related to the enniatins.
Further Information
cyclic oligopeptide Ionophore
Being a Potassium selective ionophore, valinomycin is used in specific potassium measurment electrodes.Valinomycin is an apoptosis inducer. It is used as a tool in membrane research.
Composition
Special Info
Other Fields