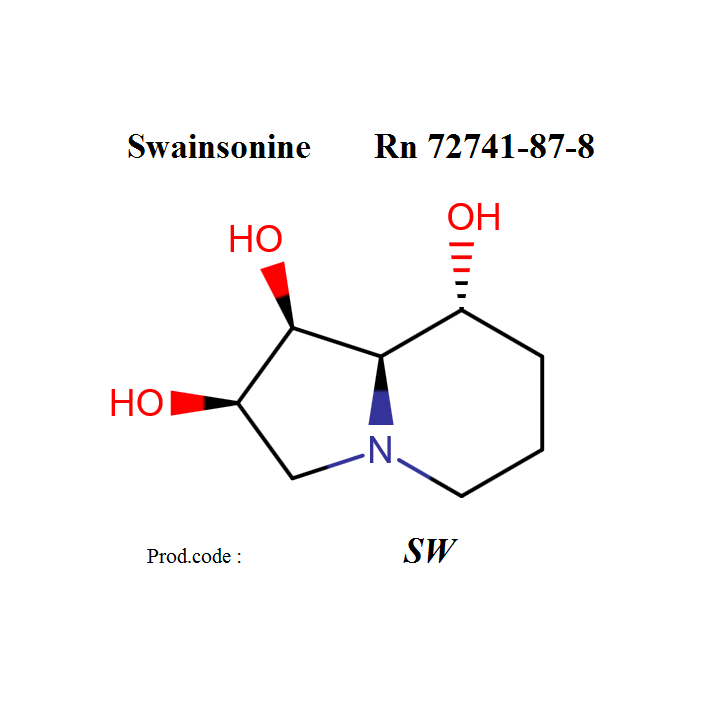
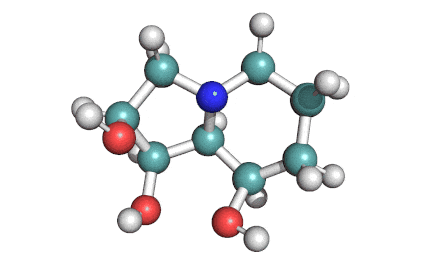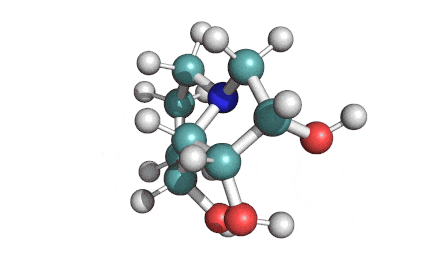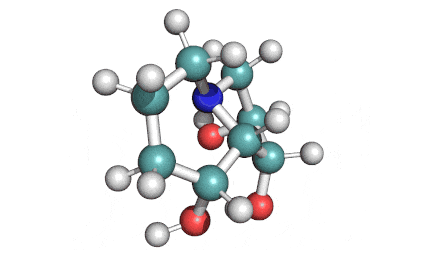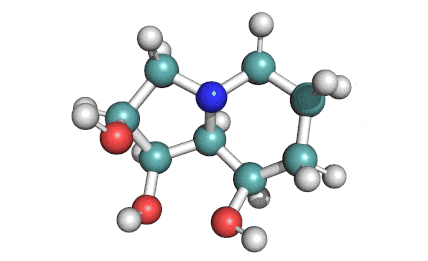
Swainsonine
Details
Specifications
Clear colorless to light yellow solution at 5 mg/ml of Methanol
Chemical identification
Synonyms:
Swainsonine;
8A,B-INDOLIZIDINE-1,2A,8B-TRIOL;
SWAINSONINE;
Tridolgosir;
(1S,2R,8R,8aR)-octahydro-1,2,8-indolizinetriol;
Chemical names:
IUPAC: (1S,2R,8R,8aR)-1,2,3,5,6,7,8,8a-octahydroindolizine-1,2,8-triol
RTECS# NM2408666
An indolizidine alkaloid from fungus Metharhizium anisopliae, a potent alpha-mannosidase inhibitor.
Swainsonine also exhibits antimetastatic, antiproliferative, and immunomodulatory activity.
Further Information
Swainsonine has a potential for treating cancers such as glioma and gastric carcinoma.
However, a phase II clinical trial of GD0039 (a hydrochloride salt of swainsonine) in 17 patients with renal carcinoma was discouraging.
Swainsonine's activity against tumors is attributed to its stimulation of macrophages.
Swainsonine also has potential uses as an adjuvant for anti-cancer drugs and other therapies in use.
In mice, swainsonine reduces the toxicity of doxorubicin, suggesting that swainsonine might enable use of higher doses of doxorubicin.
Swainsonine may promote restoration of bone marrow damaged by some types of cancer treatments.
Composition
Special Info
Other Fields