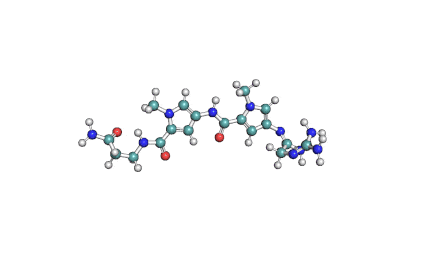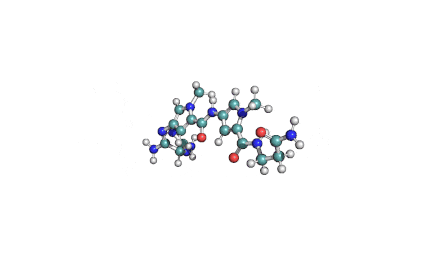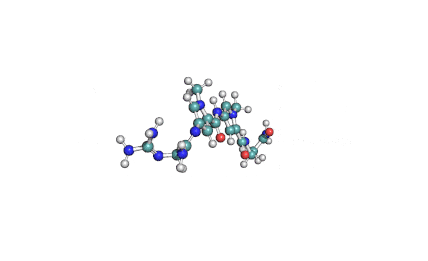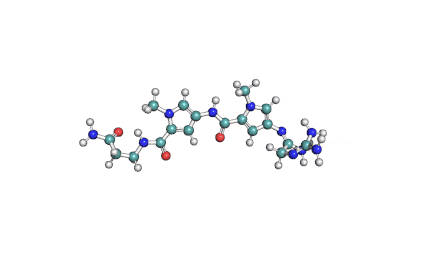
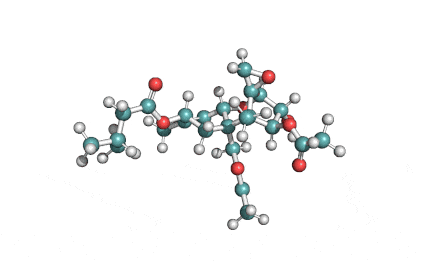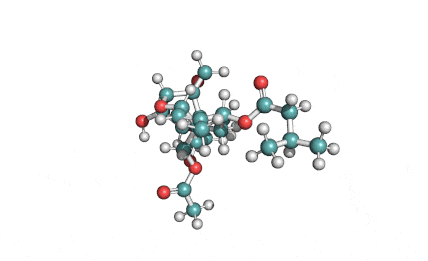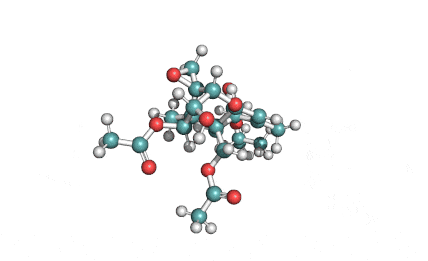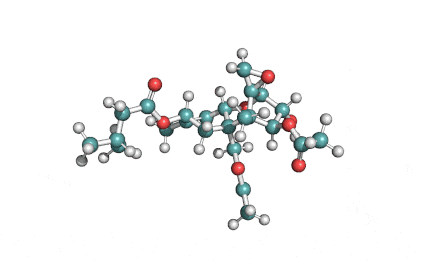
Netropsin 2HCl
Details
Specifications
Chemical identification
Synonyms:
- Congocidin
- Sinanomycin
- Netropsin
IUPAC: N-{5-[(3-amino-3-iminopropyl)carbamoyl]-1-methyl-1H-pyrrol-3-yl}-4-{[N-(diaminomethylidene)glycyl]amino}-1-methyl-1H-pyrrole-2-carboxamide
RTECS DW2974000
A basic cytotoxic polypeptide from Streptomyces netropsis. Netropsin binds to A-T sequences of DNA.
Further Information
Chemical classification:
- Pyrrole-amidine antibiotic
- Oligopetptide antibiotic
Pharmaceutic classification
- Antibacterial
- Antiviral
Composition
Supply related information
Special Info
Other Fields