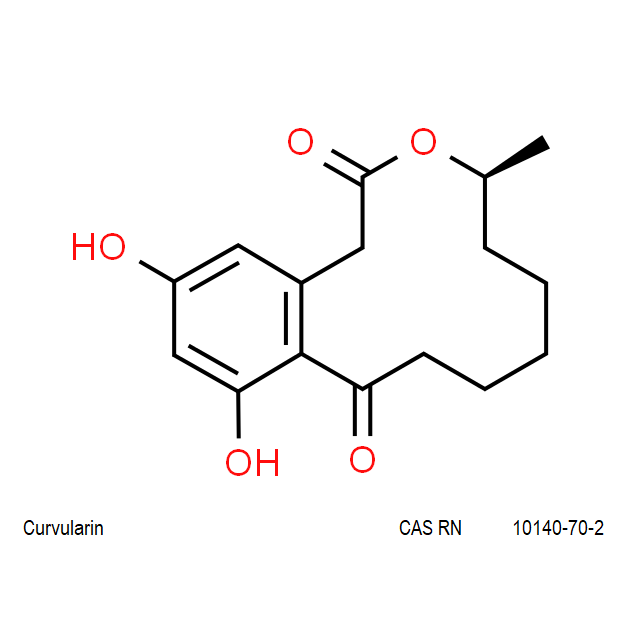
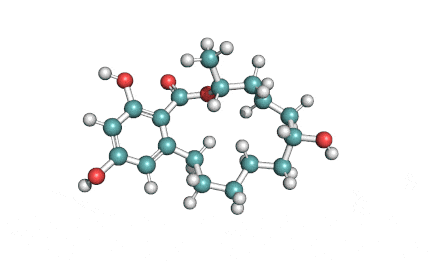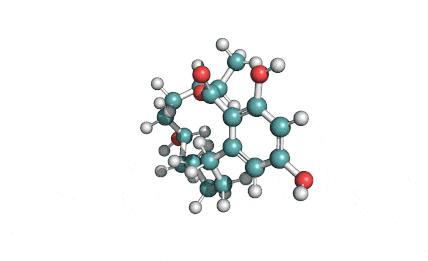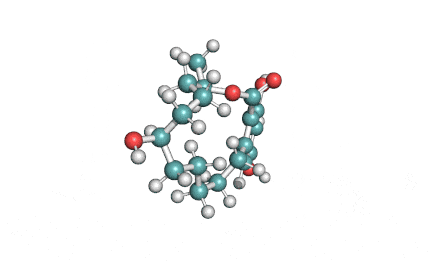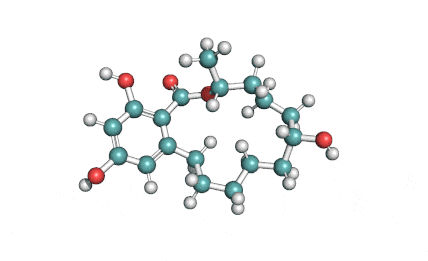
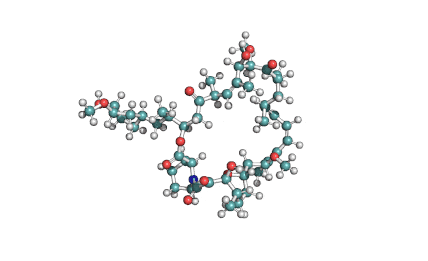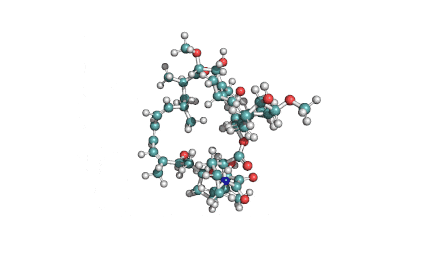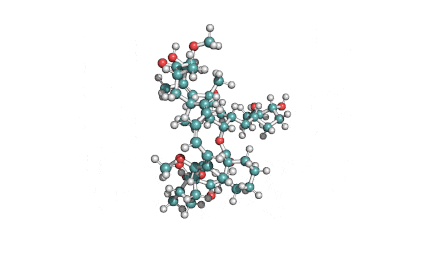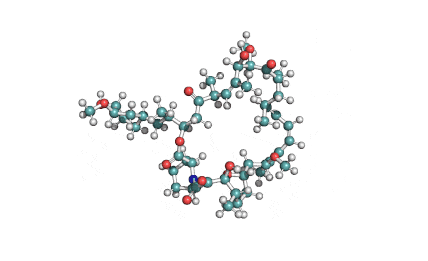
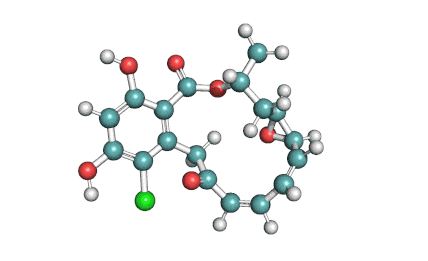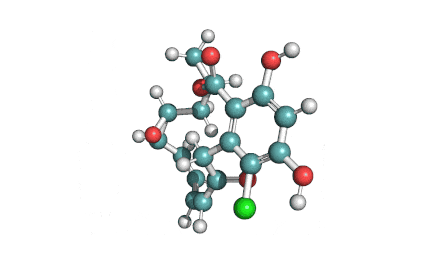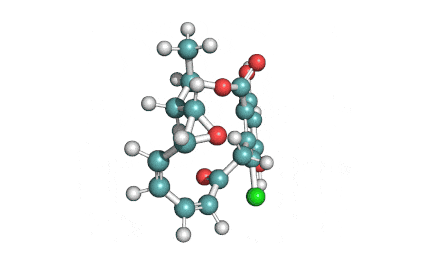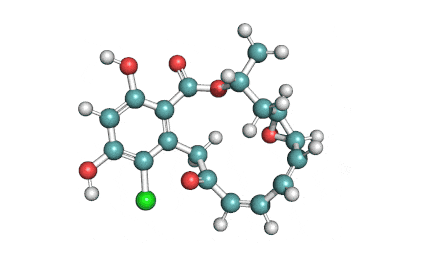
Curvularin
Details
Specifications
Chemical identification
Synonyms:
Chemical names: Curvularin;
IUPAC: (4S)-11,13-Dihydroxy-4-methyl-4,5,6,7,8,9-hexahydro-2H-3-benzoxacyclododecine-2,10(1H)-dione
RTECS n.a.
Curvularin, an antibiotic from penicillium fungus, when added to cattle feed, may promote growth and increase feed efficiency.
Further Information
Curvularin is reported to be soluble in ethanol, methanol, DMF and DMSO.
Natural antibiotic, anti-bacterial, antifungal, phytotoxic.
Composition
Special Info
Other Fields