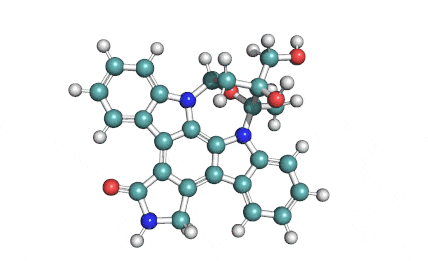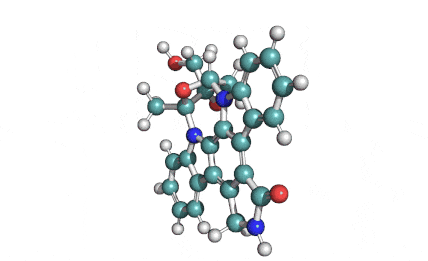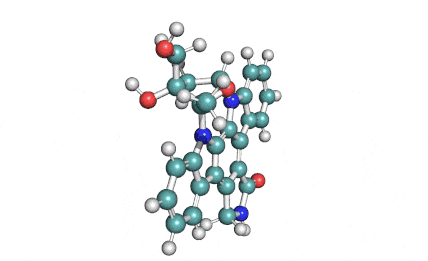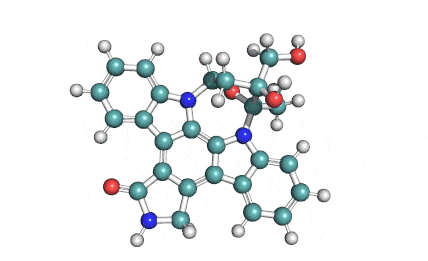
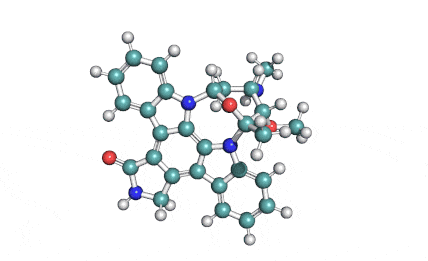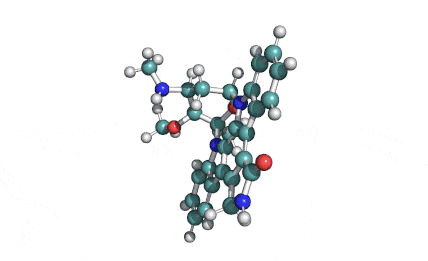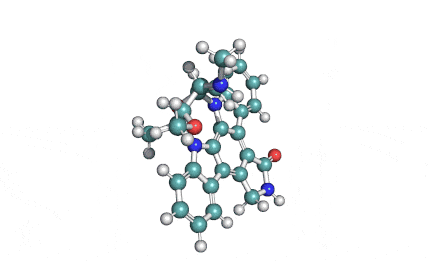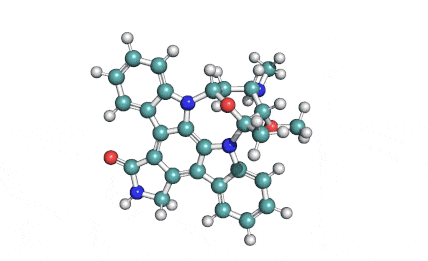
KT5555 (Lestaurtinib)
Details
Specifications
Chemical identification
IUPAC/Chemical name: (5S,6S,8R)-6-hydroxy-6-(hydroxymethyl)-5-methyl-7,8,14,15-tetrahydro-5H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacen-13(6H)-one
Synonyms
- KT5555
- Lestaurtinib
KT5555 is a multi targeted tyrosine kinase inhibitor.
Further Information
Chemical classification
- Indole alcaloids - Carbazoles - Staurosporine-derivates
Bioactivity classification
- Enzyme inhibitors
- Proteine kinase inhibitors / Tyrosine kinase inhibitors
It is used for the treatment of pancreatic cancer and acute myelogenous leukaemia (AML).
Composition
Special Info
Other Fields