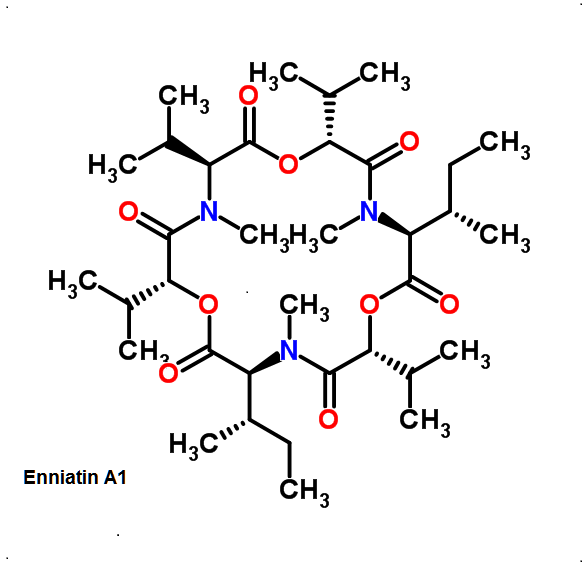
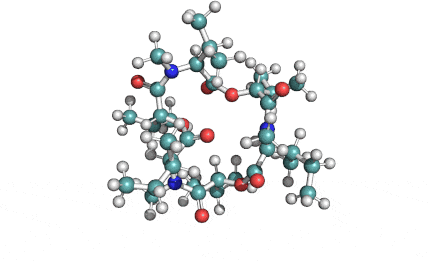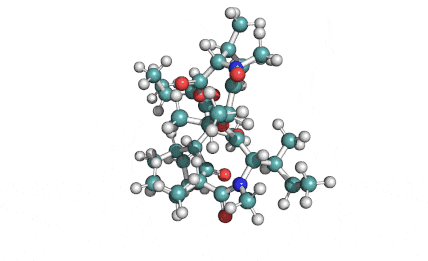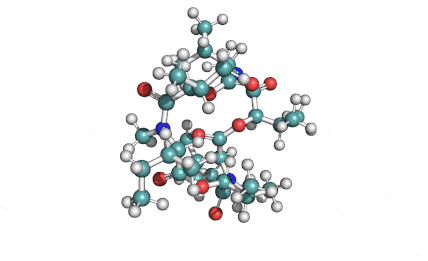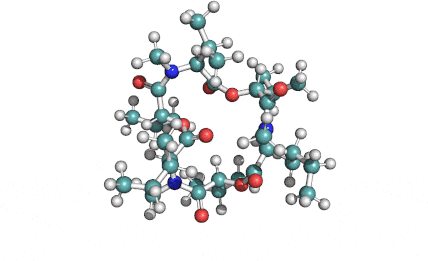
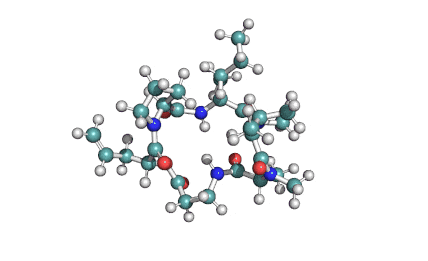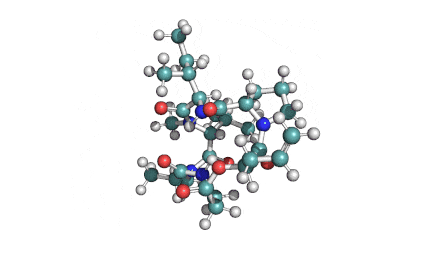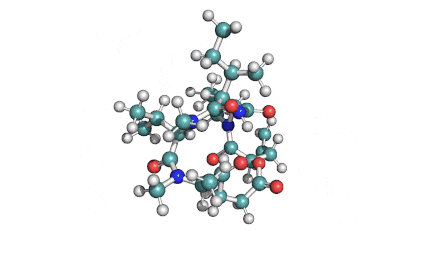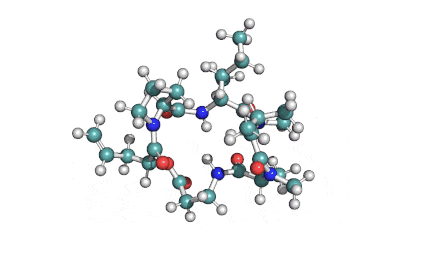
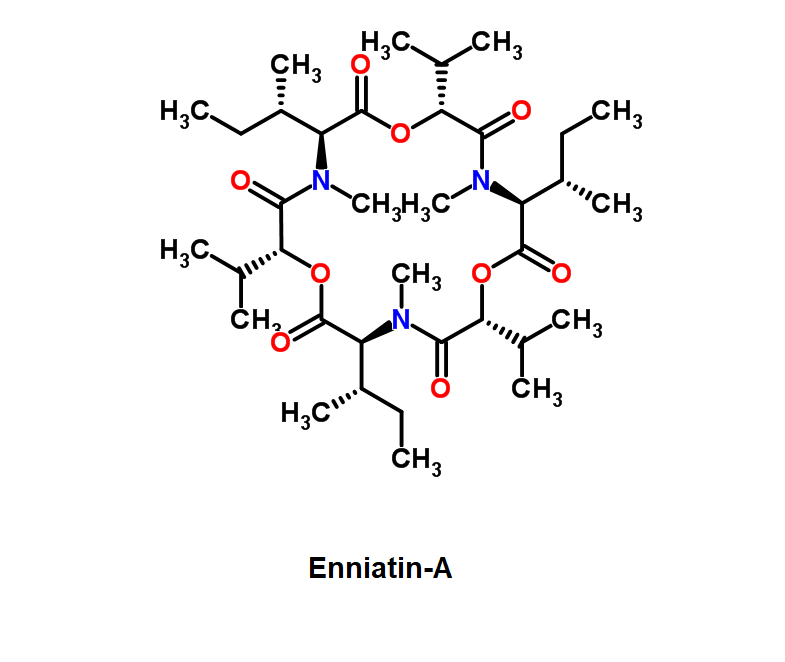
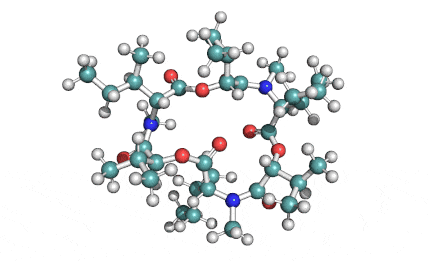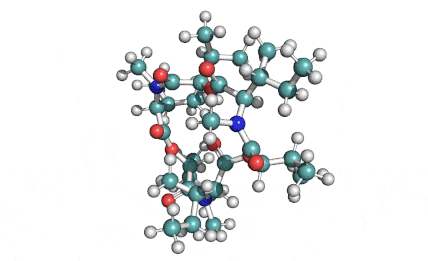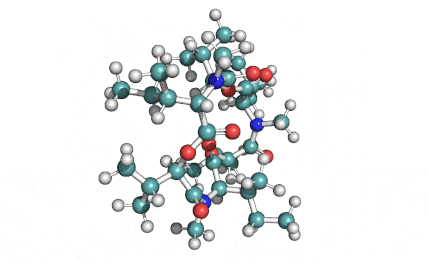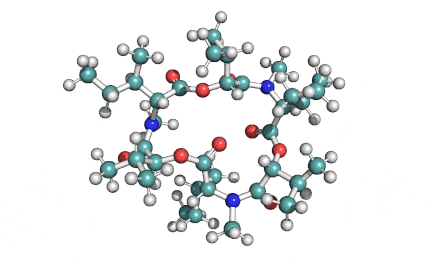
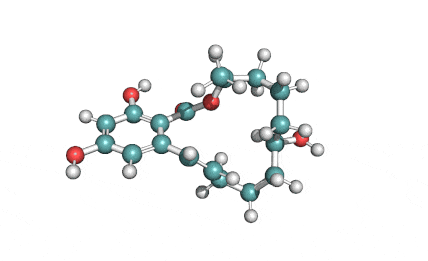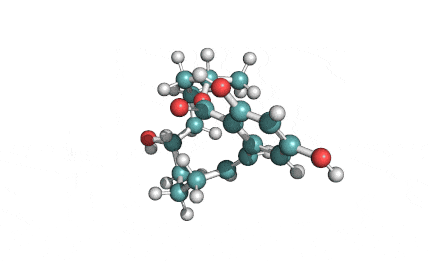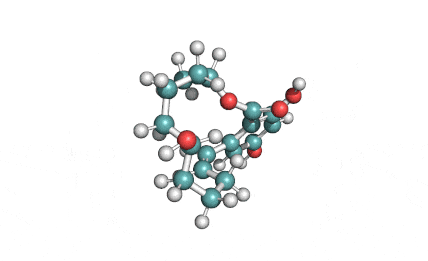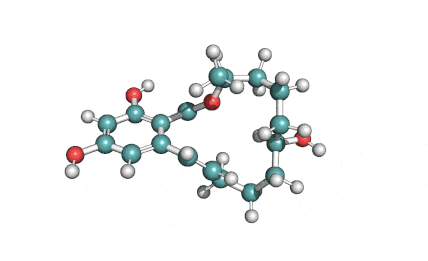
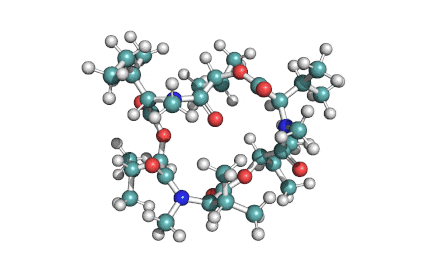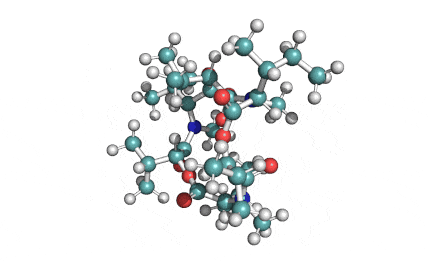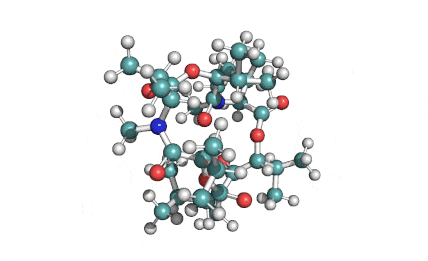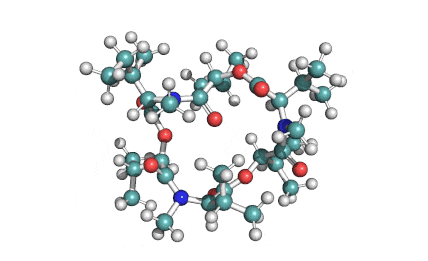
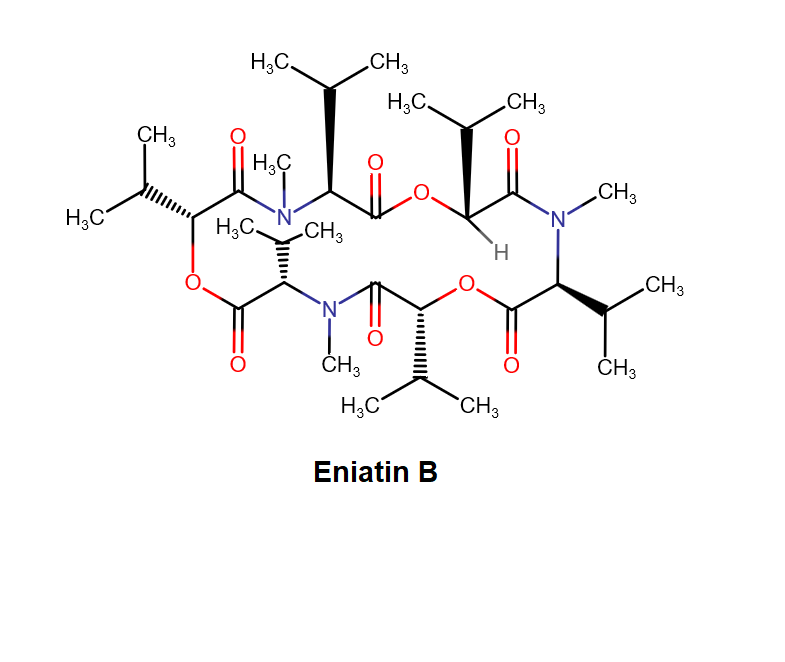
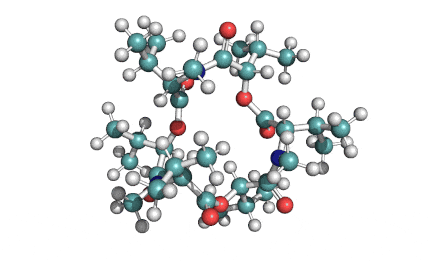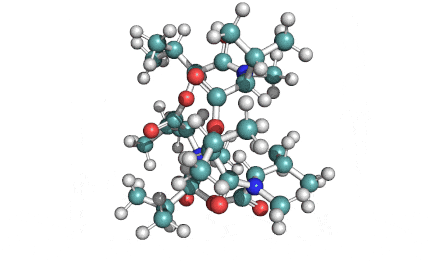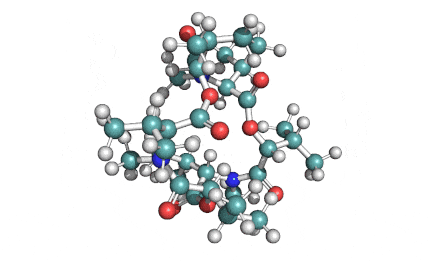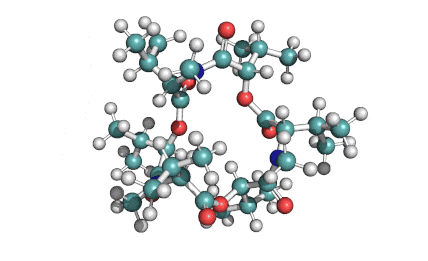
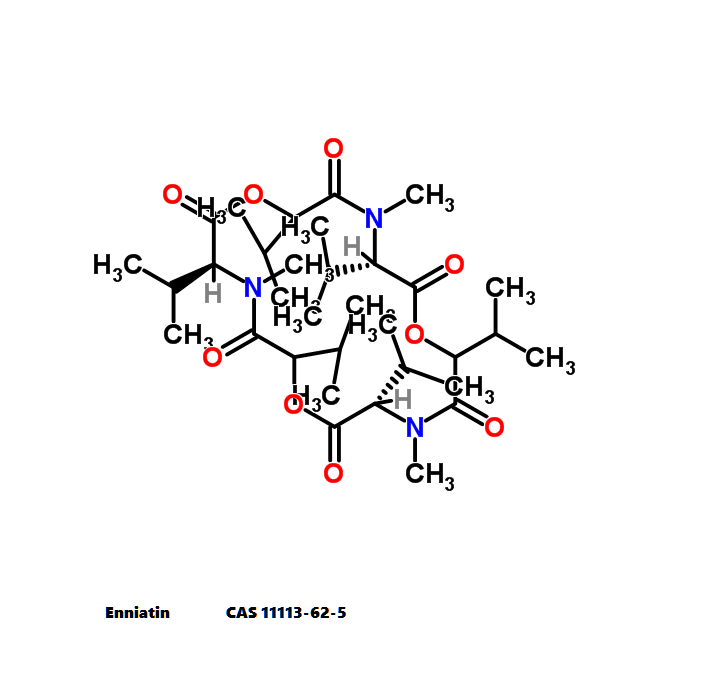
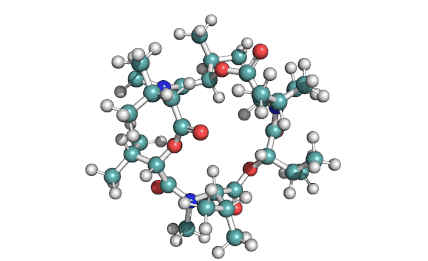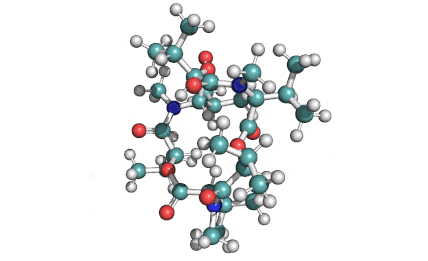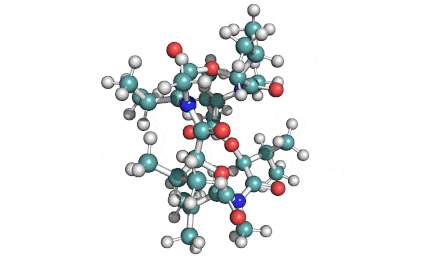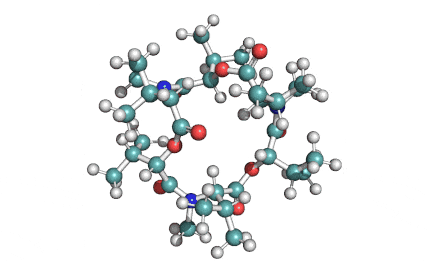
Enniatin A1
Details
Specifications
Chemical identification
Synonyms:
Chemical names: Enniatin A1
IUPAC: 3S,6R,9S,12R,15S,18R)-3,9-bis[(2S)-butan-2-yl]-4,10,16-trimethyl-6,12,15,18-tetra(propan-2-yl)-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone
IUPAC, Biological notation: cyclo[N(Me)Ile-D-OVal-N(Me)Ile-D-OVal-N(Me)Val-D-OVal]
Enniatin A1 has been found to induce apoptosis in cancer cells ...
Further Information
Chemical Classification:
Cyclodepsipeptide
Composition
Special Info
Other Fields