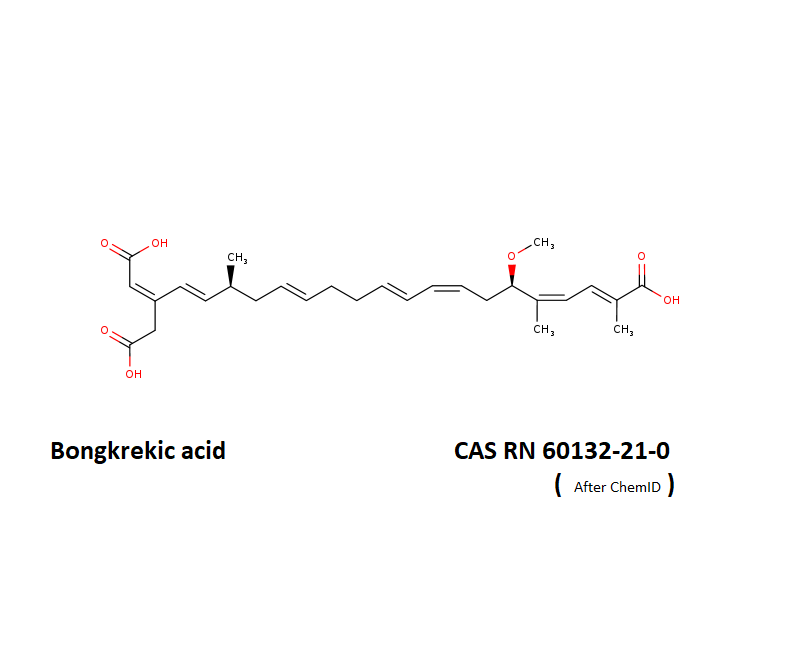
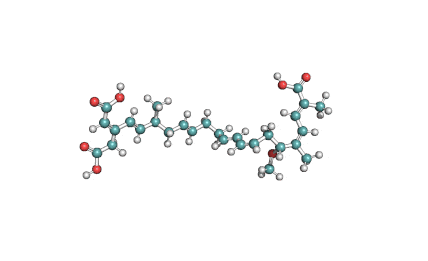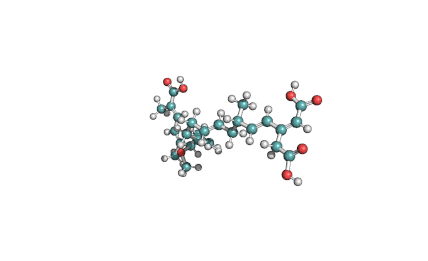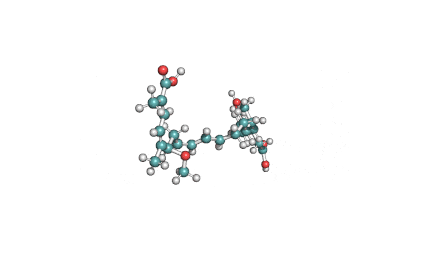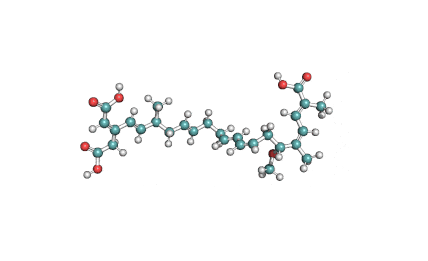
Bongkrekic acid (as solution)
Details
Specifications
Chemical identification
Synonyms:
- Bongkrekic Acid
- Flavotoxin A
Chemical names:
IUPAC:
- (6R,17S)-20-(carboxymethyl)-6-methoxy-2,5,17-trimethyldocosa-2,4,8,10,14,18,20-heptaenedioic acid
RTECS
Supplied as 1mg/ml solution dissolved in 0.01M Tris buffer pH 7.5.
Further Information
- Solubility in Water: ~ 1 mg/ml
- Solubility Notes: ~100 mg/ml in DMSO
- Chemical classification:
- Polyunsaturated tricarboxylic acid
Bioactivity:
- Glutathione transferase inhibitor
- ATP/ADP translocase inhibitor
Bongcrekic acid is an inhibitor of adenine nucleotide translocator, which inhibits apoptosis, and is thus an important tool for the mechanistic investigation of apoptosis.
Composition
Supply related information
Special Info
Other Fields