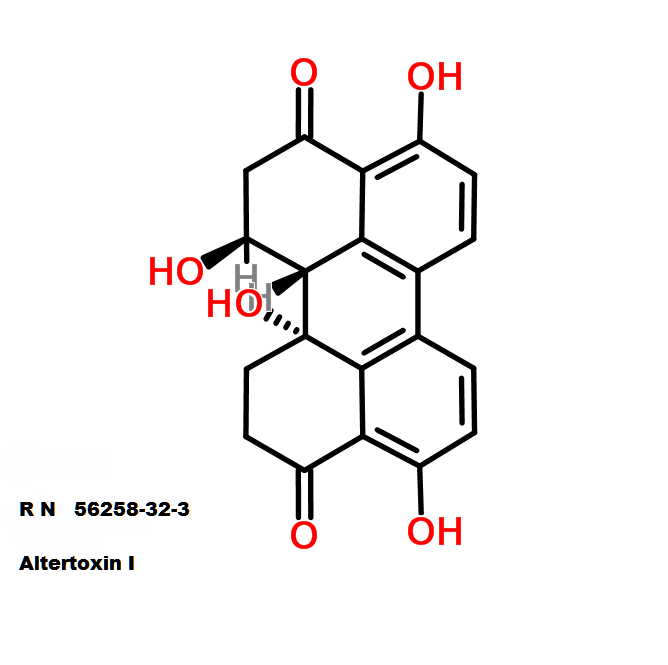
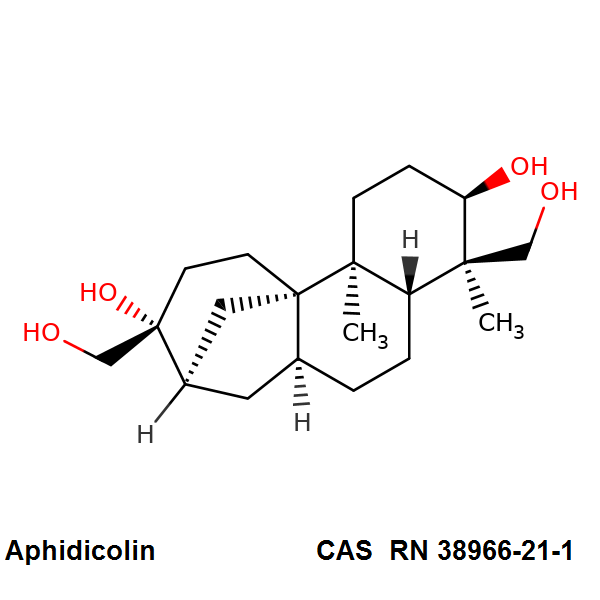
Altertoxin I
Details
Specifications
Chemical identification
Synonyms:
- (12S,12As,12Br)-4,9,12,12B-Tetrahydroxy-2,11,12,12A-Tetrahydro-1H-Perylene-3,10-Quinone;
- Altertoxin I
- Altertoxin-I
Chemical names:
IUPAC:
- (12S,12aS,12bR)-4,9,12,12b-tetrahydroxy-2,11,12,12a-tetrahydro-1H-perylene-3,10-dione
RTECS# : BC9625200
Altertoxin-1, a natural mycotoxin from Alternaria fungi, an important contaminant in cereals, vegetables.
Further Information
Altertoxin I (and other altertoxins) inhibit the HIV replication.
Composition
Special Info
Other Fields