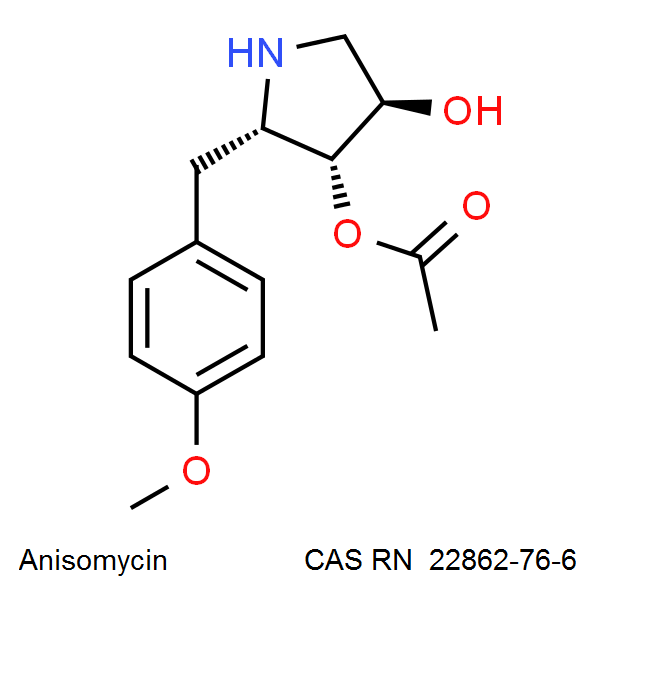
Anisomycin
Details
Specifications
Chemical identification
Synonyms : Flagecidin
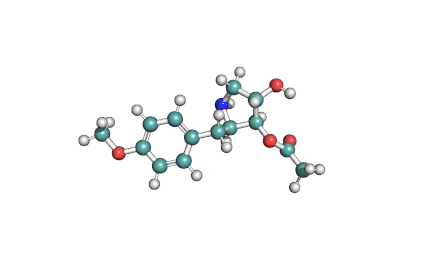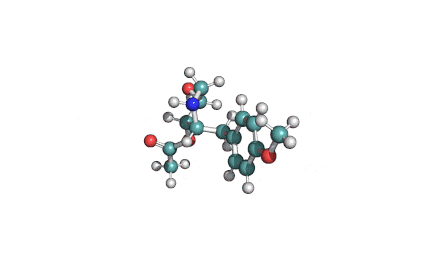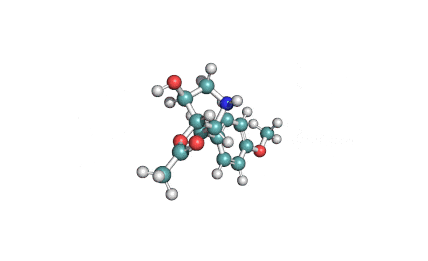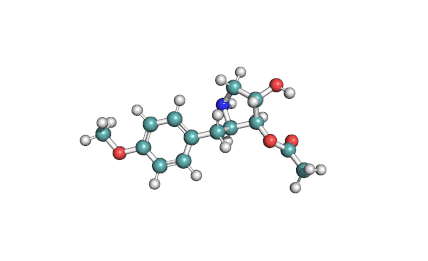
IUPAC Name: [(2R,3S,4S)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate
Anisomycin is a protein synthesis inhibitor. It binds to the 60S ribosomal subunits and prevents the formation of peptide bond, and consequently, the chain elongation.
Further Information
Anisomycin is soluble in DMSO, Ethyl-Acetate, Dichloromethane. Slightly soluble in water
Biological action classification
- Protein Synthesis Inhibitor
- Nucleic Acid Synthesis Inhibitor
Chemical classification
- Pyrrolinin class antibiotic
Originally discovered by Sobin and Tanner in 1954, Anisomycin was identified as an antibiotic active against protozoa such as Trichomonas and fungi and yeasts, and was considered as a potential human anti-candidal and anti-amoebic drug. Sobin, B. A., and F. W. Tanner, Jr. 1954. Anisomycin, a new antiprotozoa antibiotic. J. Am. Chem. Soc. 76:4053. As such, Anisomycin was put by GONZALEZ CONSTANDSE to clinical trials in 1955: Anisomycin in intestinal amebiasis; study of 30 clinical casesPrensa Med Mex. 1956 Sep-Dec;21(7-10):114-5 Anisomycin is also proposed as a potential psychiatric drug, as it is shown to affect protein synthesis an Amygdala, a brain part involved in memory(1). The earliest usage of Anisomycin in research on memory biochemistry was made by Jonec and Walsterlain in 1979 . Again, this property of Anisomycin is ascribed to its ability to inhibit protein synthesis. Effect of inhibitors of protein synthesis on the development of kindled seizures in rats.Jonec V, Walsterlain CG.Exp Neurol. 1979 Dec;66(3):524-32 Recently, publications appear showing the immunomodulating properties on Anisomycin. This discovery leads to researches on the potential use of Anisomycin in transplantation: Anisomycin inhibits the behaviors of T cells and the allogeneic skin transplantation in mice.Xing F et al,J Immunother. 2008 Nov-Dec;31(9):858-70 In the field of cell-signaling research, Anisomycin is used as an activator of kinase cascades in mammalian cells, especially the stress-activated Protein Kinase (SAPK2/p38MAPK) and p46/54JNK Anisomycin is a selective ingredientof Martin Lewis Agar, a bacteriological solid medium used for isolation of pathogenic Neisseria species. Martin, J. E., Jr., and J. S. Lewis. 1977. Anisomycin: improved antimycotic activity in modified Thayer-Martin medium. Public Health Lab. 35:53-62
Composition
Special Info
Other Fields