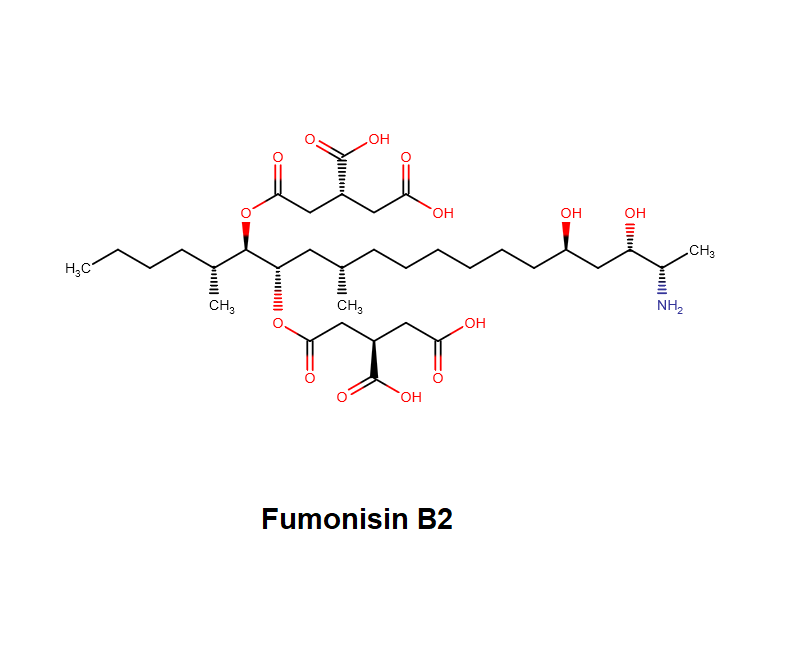
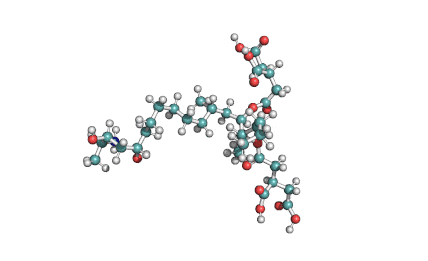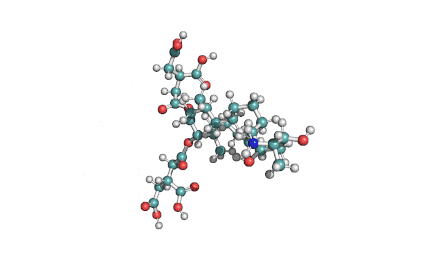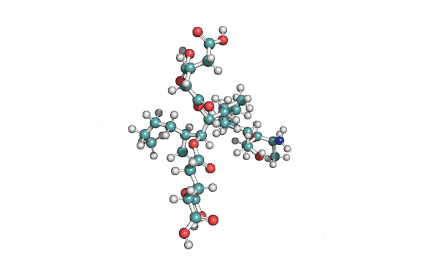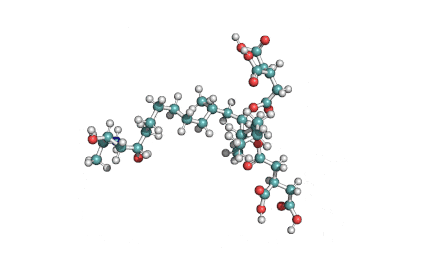
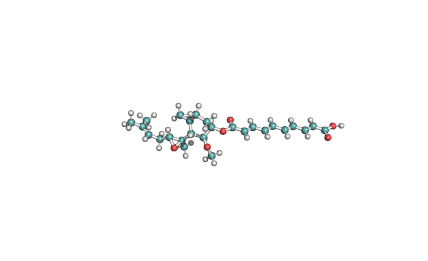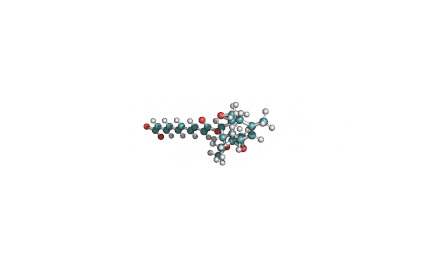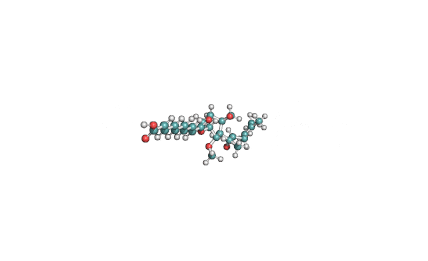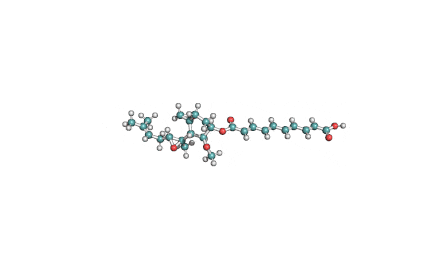
Fumonisin B2
Details
Specifications
Chemical identification
RTECS: TZ8335000
IUPAC name: (2R,2'R)-2,2'-{[(5R,6S,7S,9S,16R,18S,19S)-19-amino-16,18-dihydroxy-5,9-dimethylicosane-6,7-diyl]bis[oxy(2-oxoethane-2,1-diyl)]}disuccinic acid
Structural analog of Fumonisin B1. Fumonisin B2 is more cytotoxic than Fumonisin B1.
Fumonisin B2 inhibits sphingosine acyl-transterase.
Further Information
Methanol
- Isoflavonoid
- Phytoestrogen.
- Sphingosine acyl-transterase inhibitor
sphingosine acyl-transterase inhibitor
Composition
Special Info
Other Fields