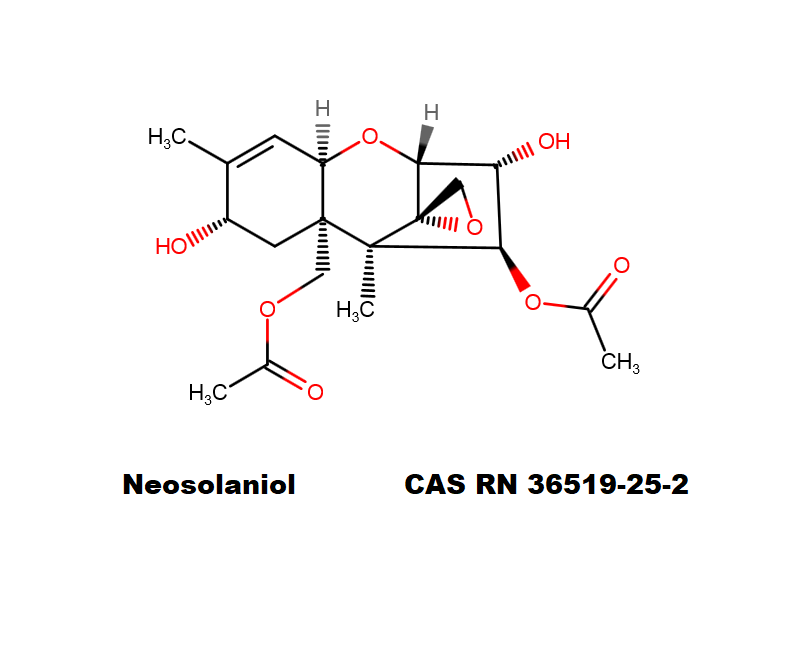
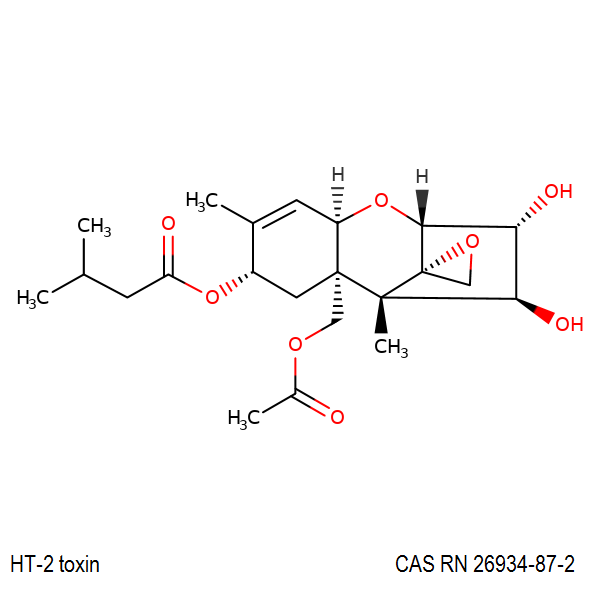
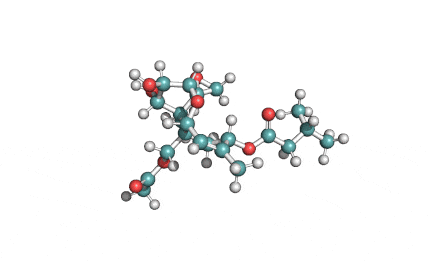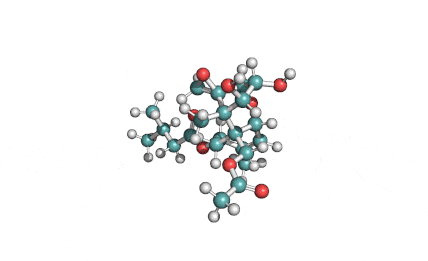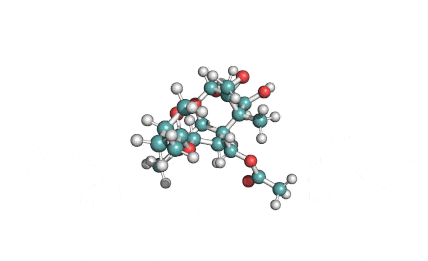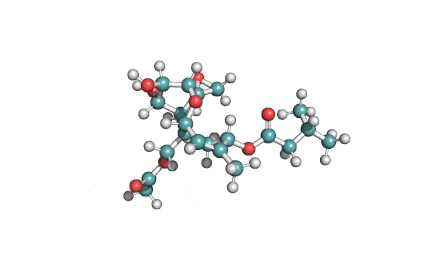
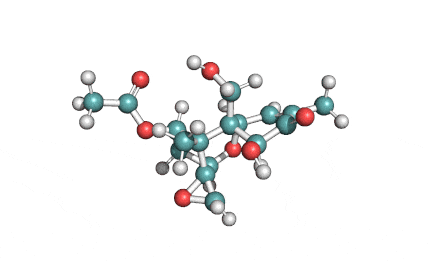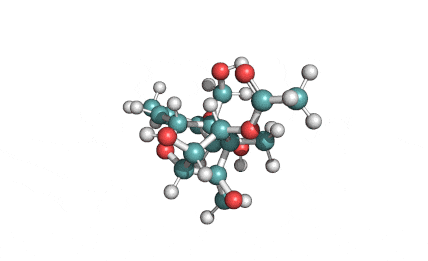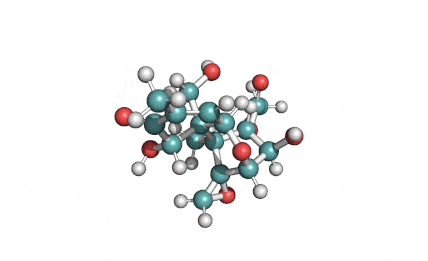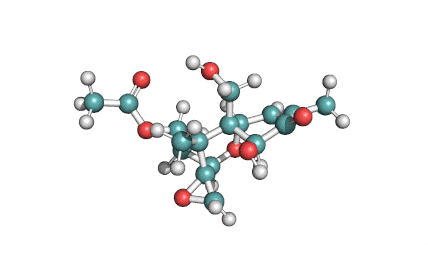
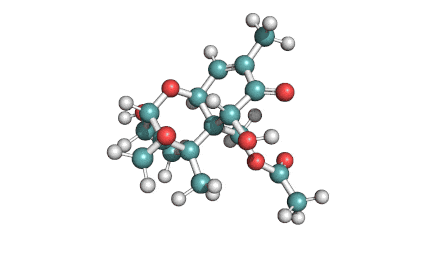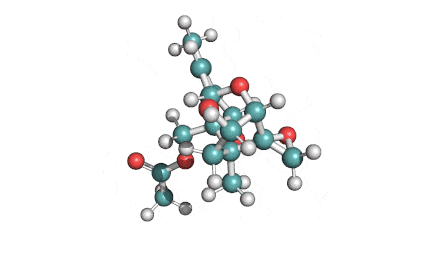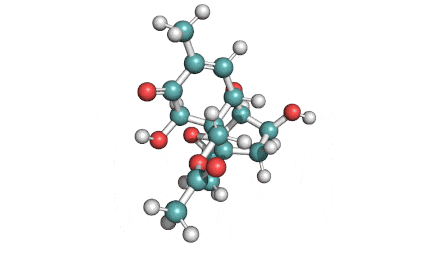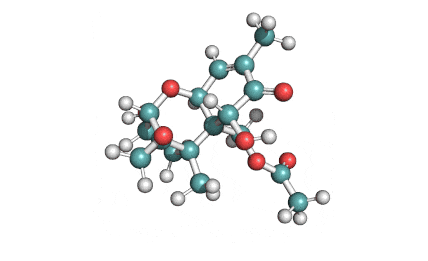
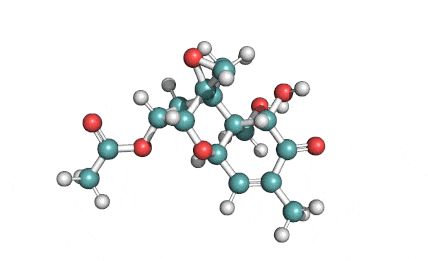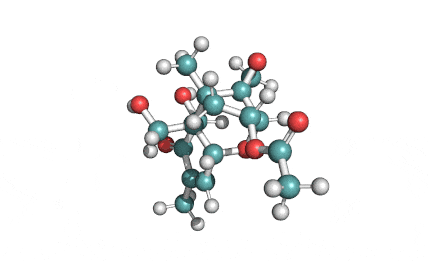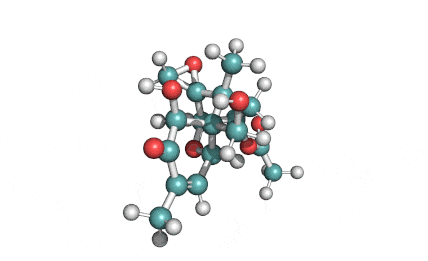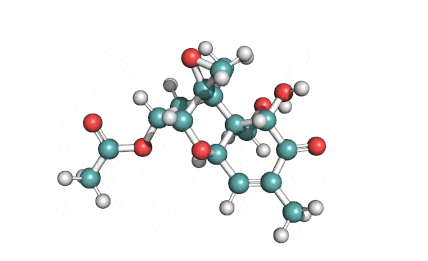
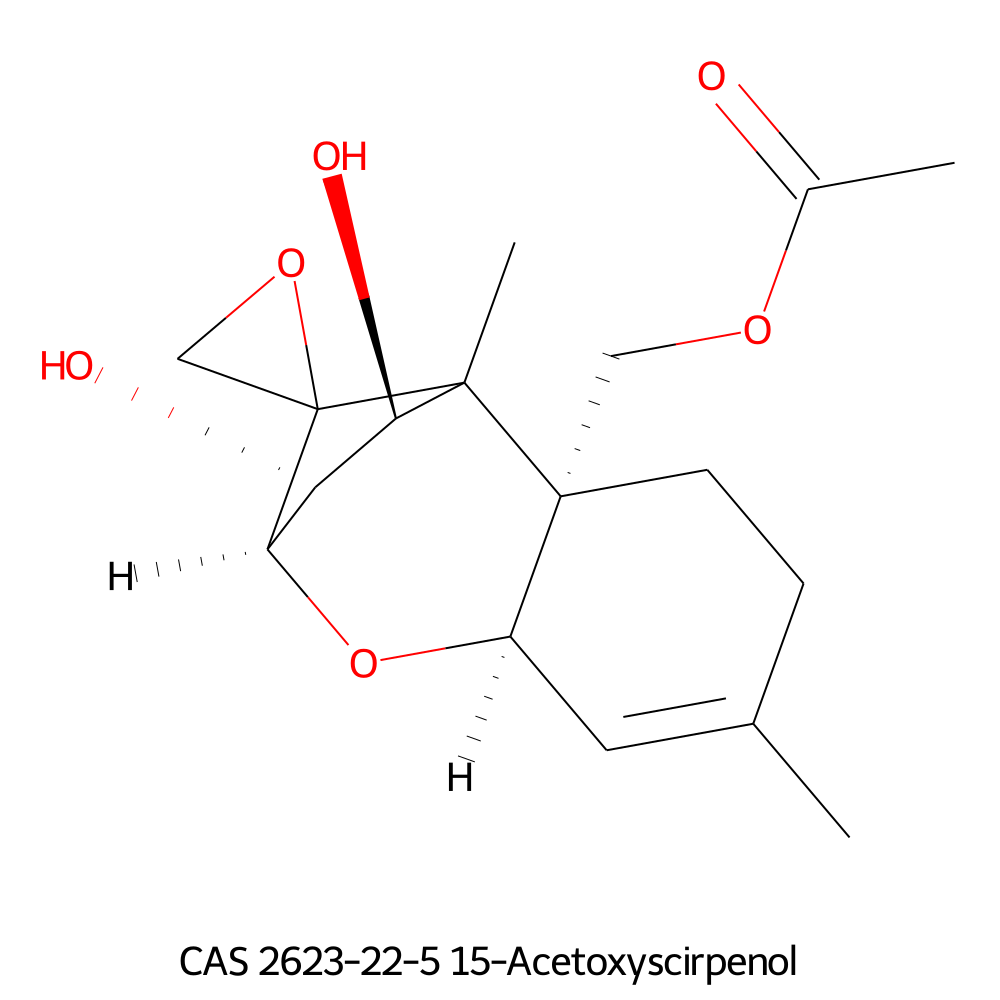
Neosolaniol
Details
Specifications
Chemical identification
Synonyms:
- Neosolaniol
- Solaniol
- 8-Hydroxydiacetoxyscirpenol
- Trichothec-9-ene-3-alpha,4-beta,8-alpha,15-tetrol, 12,13-epoxy-, 4,15-diacetate
RTECS: YD0080000
Type A trichothecene mycotoxin
Further Information
Soluble in moderately polar solvents, such as chloroform, diethyl ether, ethyl acetate, and acetone
trichothecene mycotoxin
All trichocenes have strong impact on the health due to their immunosuppressive properties
Composition
Special Info
Other Fields