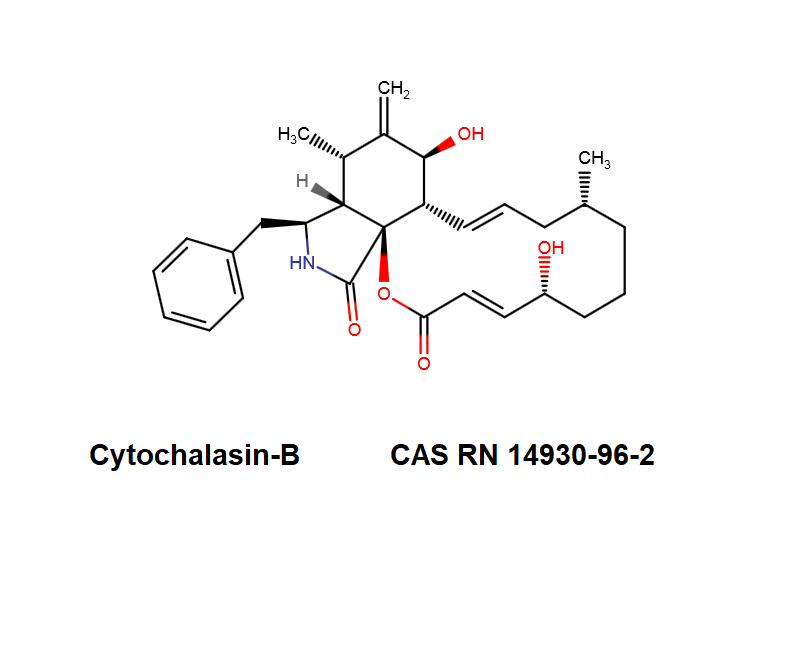
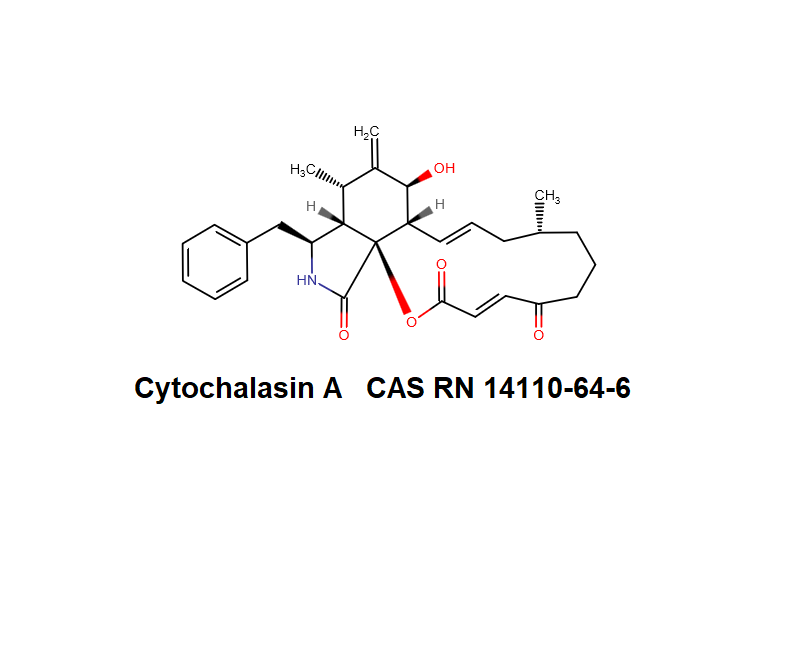
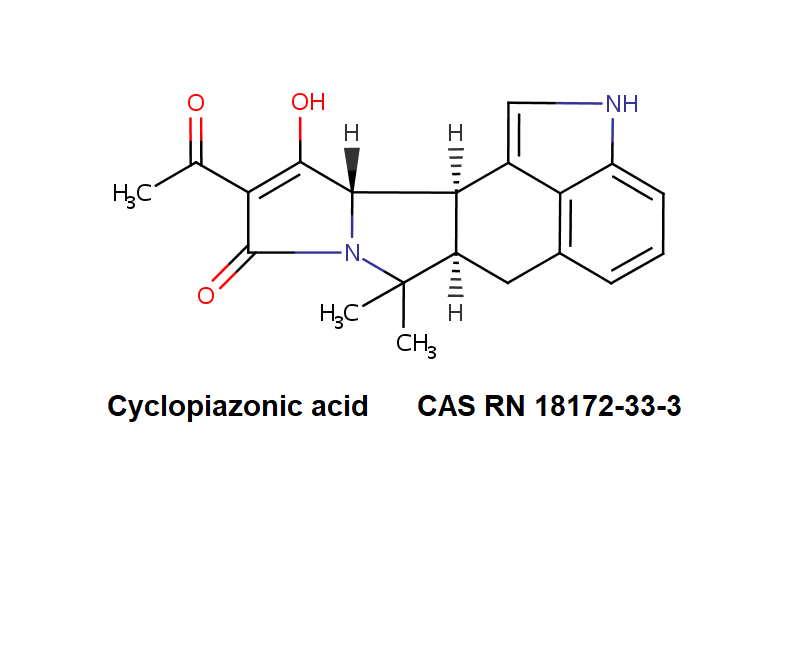
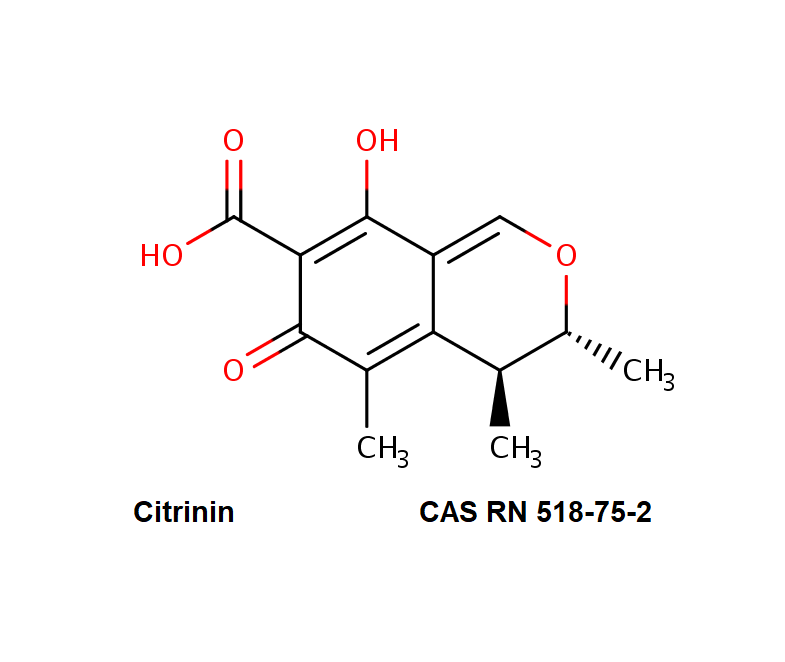
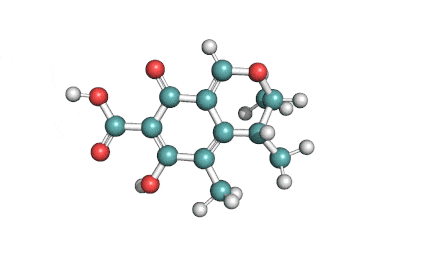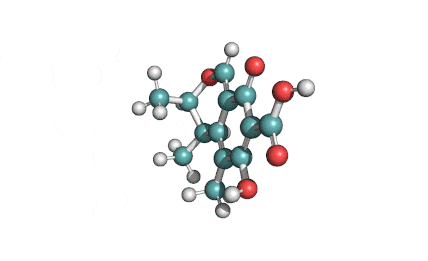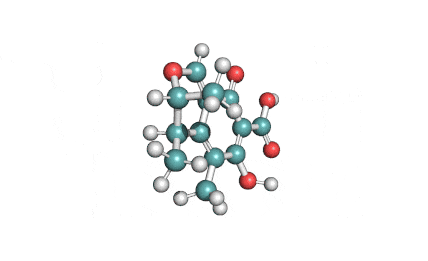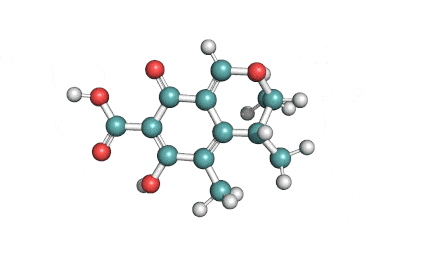
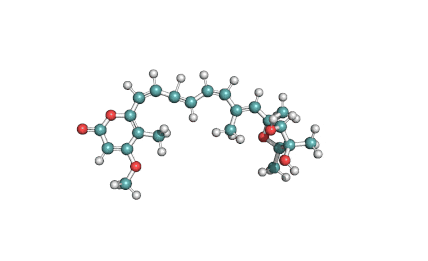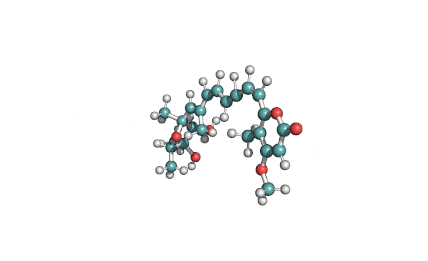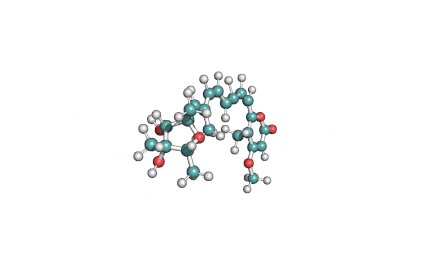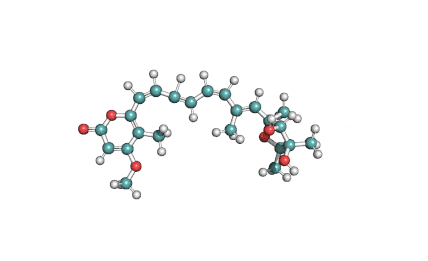
Cytochalasin C
Details
Specifications
Chemical identification
RTECS: HA5300500
A fungal metabolite that acts as a potent inhibitor of actin filament and contractile microfilaments.
Further Information
Dichloromethane , Ethyl acetate
Macrolide indol mycotoxinCytochalasinActin inhibitor
Cytochalasins are used as tools in cytological research, and in the field of actin polymerisation
Composition
Special Info
Other Fields