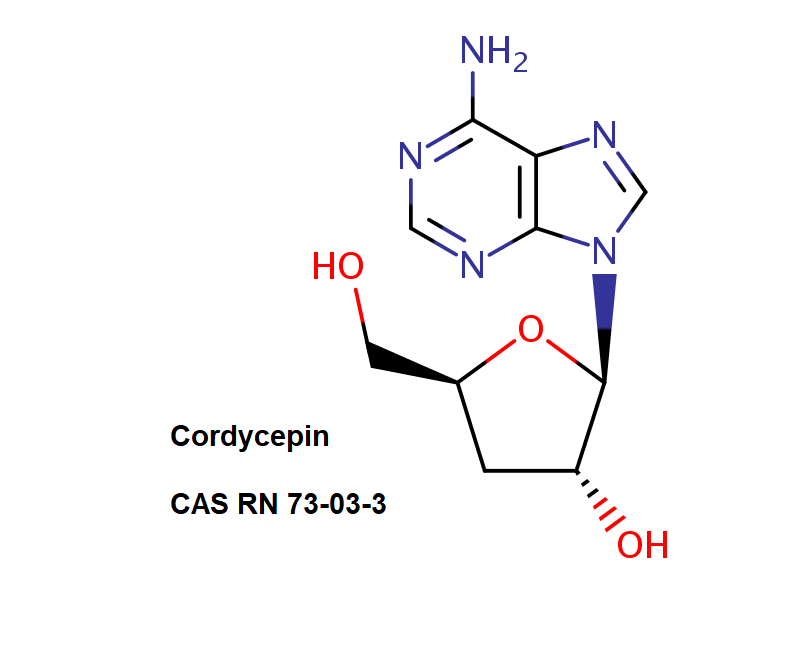
Cordycepin
Details
Specifications
Chemical identification
Systematic name:
3'-Deoxyadenosine
RTECS:
AU7358610;
Adenosine analog which occurs naturally in Cordyceps militaris, a mushroom known in traditional medicine.
Further Information
Cordycepin is soluble in water, methanol, ethanol, DMSO
Natural Nucleoside antibiotic.
Purine nucleoside
Cordycepin inhibits growth of various tumor cells in vitro. It may be converted to 3'-deoxyadenosine triphosphate , thus inhibiting ATP-dependent DNA synthesis. Cordycepin is used in the study of messenger RNA transcription.
Composition
Special Info
Other Fields