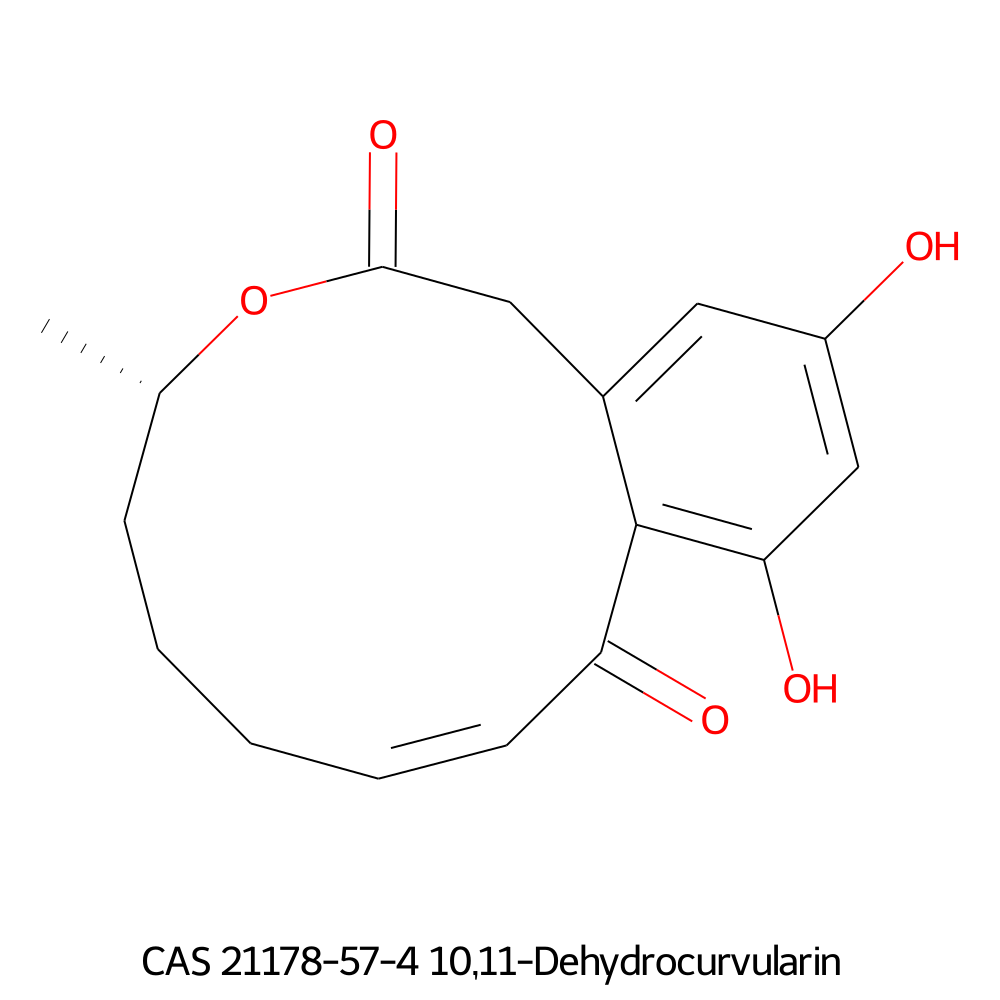
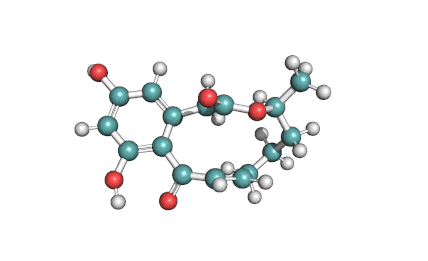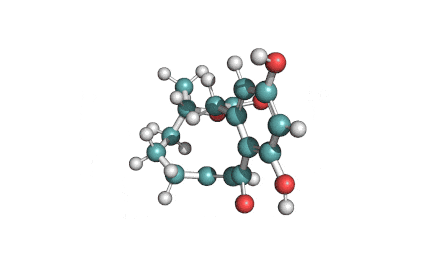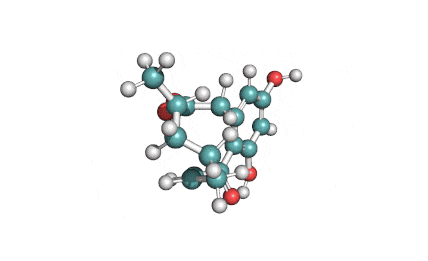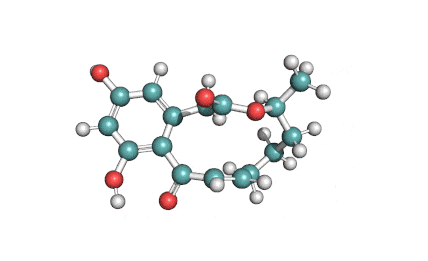
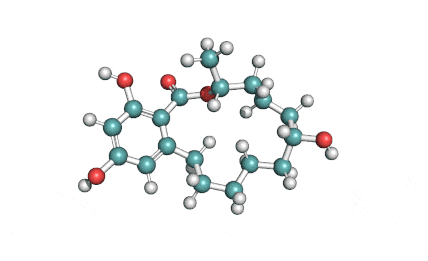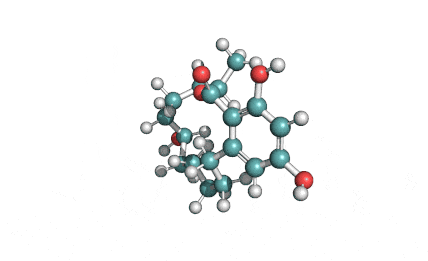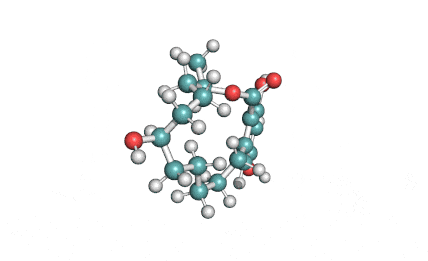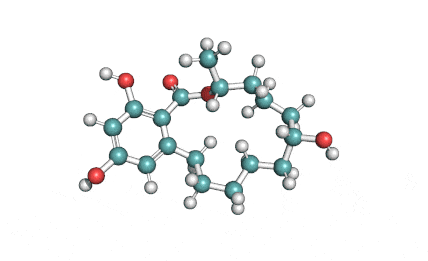
10,11-Dehydrocurvularin
Details
Specifications
Chemical identification
Synonyms:
- Trans-Dehydrocurvularin
- Dehydrocurvularin
- 10,11-dehydrocurvularin
- alpha,beta-Dehydrocurvularin
- 1095588-70-7
Comment: There are two CAS Registration numbers, corresponding to the name Dehydrocurvularin. 1095588-70-7, 21178-57-4 These numbers are not "synonyms." The represent two stereo isomers.
Chemical names: 10,11-Dehydrocurvularin
IUPAC: (5S,9E)-13,15-dihydroxy-5-methyl-4-oxabicyclo[10.4.0]hexadeca-1(12),9,13,15-tetraene-3,11-dione
RTECS#
10,11-Dehydrocurvularin is an inhibitor of cell division shown to have antimalarial activity, antibacterial proprerties.
Further Information
Natural antibiotic, anti-bacterial, antifungal, phytotoxic.
Composition
Supply related information
Special Info
Other Fields