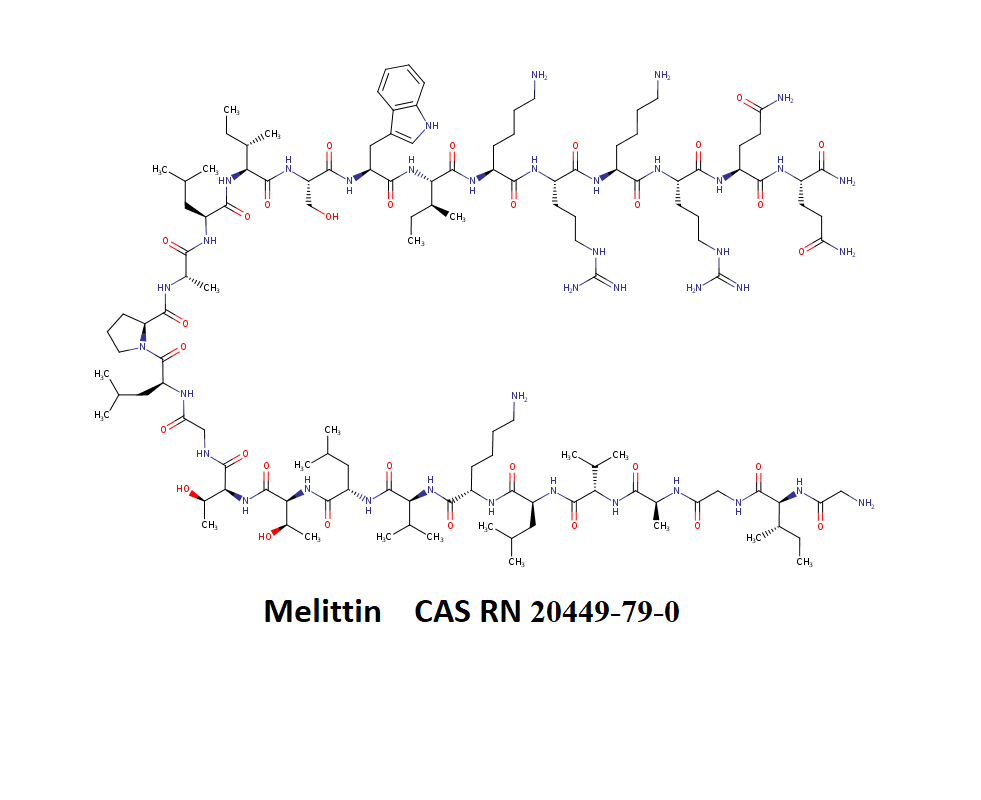
Melittin
Details
Specifications
Chemical identification
Synonyms:
- Forapine
- Honeybee melittin
- Bee venom melittin
- Melittin I
Chemical names:
IUPAC:
H-Gly-Ile-Gly-Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro-Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-Gln-NH
RTECS#
Melittin is a pore-forming toxic 26-amino-acid polypeptide that occurs in the venom of honey bees. Melittin constitutes about 40% to 60% (by weight) of that venom.
Further Information
Composition
Supply related information
Other Fields



![Mitomycin C [1mg Mitomycin C + 24mg NaCl] Mitomycin C [1mg Mitomycin C + 24mg NaCl]](/sites/default/files/2024-02/Mitomycin-C%20NaCL%20Mix.png)