Nigericin
Details
Specifications
Chemical identification
Polyetherin A; Azalomycin M; Helixin C ;
RTECS QT6825000
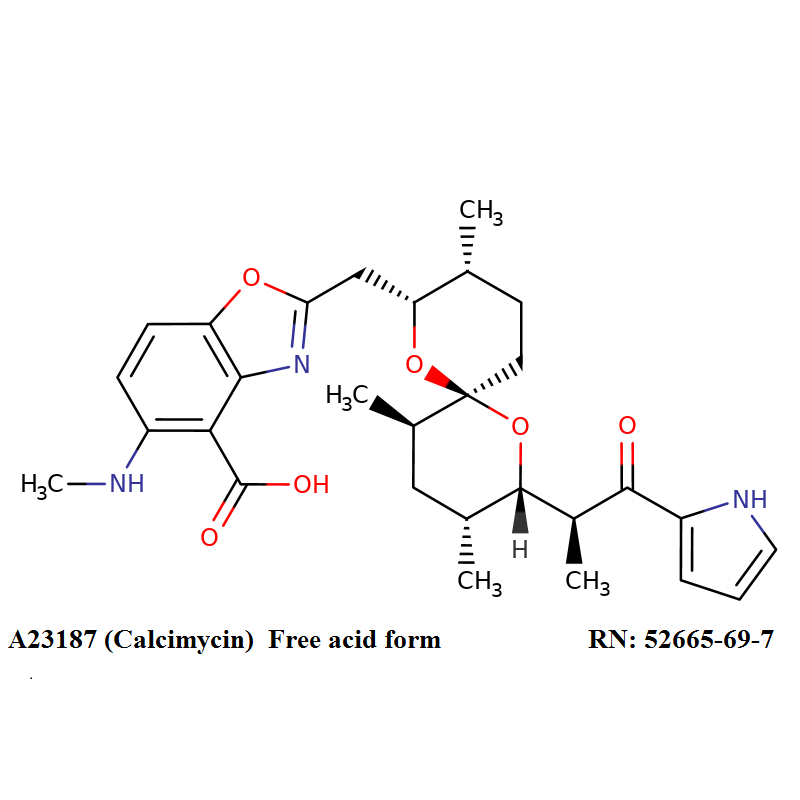
Nigericin is a polyether antibiotic which affects ion transport and ATPase activity in mitochondria.
Nigericin is produced by Streptomyces hygroscopicus. Nigericin, an ionophore and antibiotic, is supplied as a sodium salt.
Nigericin is one of the compounds successfully tested as a feed additive, displaying a significant effect on milk production in cattle. In vitro, nigericin has broad biological activity against Gram positive bacteria, fungi, tumor cell lines and some viruses, including HIV.
Further Information
DMSO, Ethanol, petrol ether. Not soluble in water
Disrupts the membrane potential and stimulates ATPase activity in mitochondria.